

on this page
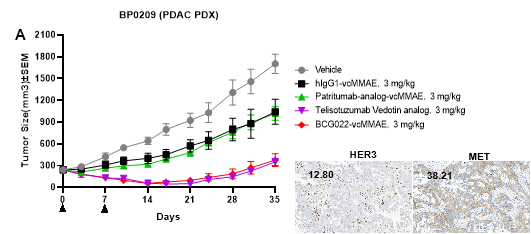
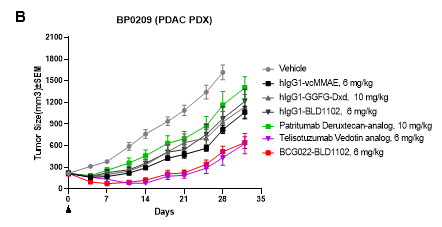
(A,B) Efficacy of BCG022-vcMMAE (DAR 4) and BCG022-BLD1102 (DAR 8) in a HER3-low PDAC PDX model. Both BCG022-BLD1102 and BCG022-vcMMAE showed efficacy in controlling growth of BP0209 tumors, with activity superior to patritumab ADCs and comparable to that of telisotuzumab ADCs. H-score values, (histochemical scores) are labelled for BP0209.
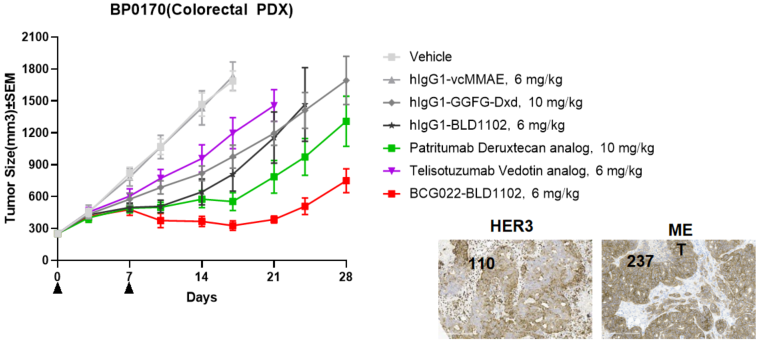
BCG022-BLD1102 showed greater anti-tumor activity compared to benchmark ADCs in a colorectal PDX model with moderate HER3 expression. Benchmark ADCs, including the patritumab deruxtecan anolog at a higher dose or telisotuzumab vedotin analog at an equivalent dose, showed weak or no activity. H-score values, (histochemical scores) are labelled for BP0170.
HER3 and MET expression act as bypass resistance mechanisms, conferring resistance to multiple therapeutic agents, such as EGFR TKI treatment. HER3 and MET are also co-expressed at high prevalence in multiple tumor types, including gastric, colorectal, breast, and non-small-cell lung cancer (NSCLC). In addition, HER3 and MET are frequently overexpressed in liver metastases from patients with colorectal cancer, indicating that targeting both targets may provide clinical benefit.
Learn more about the RenLite and ADC platform.