

Biocytogen has established a model of MOG-induced EAE disease, which can be used for the efficacy evaluation of MS-related drugs.
on this page
Multiple sclerosis (MS) is a chronic inflammatory disease of the central nervous system that can lead to encephalitis and demyelination. MS is considered an autoimmune disease with symptoms including muscle stiffness and paralysis, visual disturbance and blindness, sensory loss, and ataxia, with features of recurrent recurrence. There are many different animal models of MS, among which the experimental autoimmune encephalomyelitis (EAE) model is widely used in the study of multiple sclerosis due to its similar pathological features of inflammation and demyelination as MS.
In rodents such as mice and rats, immune immunity to generate EAE models can be generated using spinal cord homogenates, purified myelin sheaths, myelin proteins (myelin basic protein ,MBP; proteolipoprotein ,PLP; and myelin oligodendrocyte glycoprotein ,MOG), or peptides of these proteins. This may be due to myelin-specific T cells being activated peripheramally, crossing the blood-brain barrier into the central nervous system and being reactivated, triggering a cascade of inflammatory responses leading to demyelination and axonal cell apoptosis, ultimately leading to nerve damage and loss of function. Clinical symptoms are assessed using a standardized scoring system that measures the degree of disease induction, with local demyelination and inflammatory leukocyte infiltration visible in pathologic tissue stained sections.
Biocytogen has established a model of MOG-induced EAE disease, which can be used for the efficacy evaluation of MS-related drugs.
Modeling method: Animal receive MOG35-55/CFA emulsion (s.c.) on flank (blue point) or neck and buttocks (red point) on day 0, PTX (i.p.) are injected to mice 2h and 24h after MOG35-55/CFA emulsion injection. Body weight and EAE score are recorded every 2 days. lumbar enlargement of the spinal cord are collected for HE and LFB at the end.
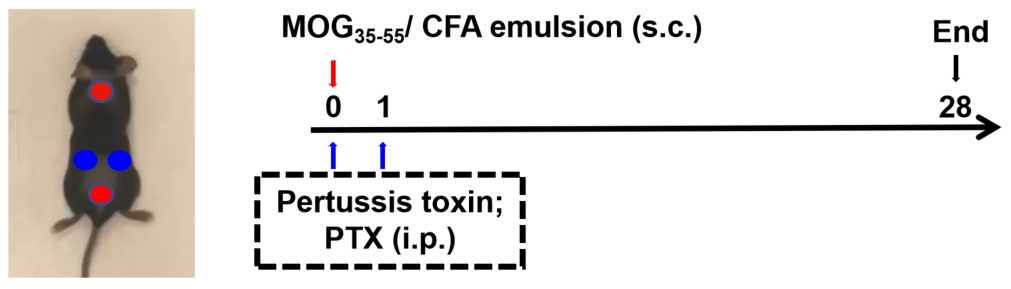
Animal: C57BL/6, B-hCD40hFcRn mice, 8-10 week, female
Detaction indicator: Body weight and clinical score (every two days) H&E, Luxol Fast Blue (LFB) staining
| Readout | ||
| Included tests | Phenotype | Body weight |
| Clinical score | ||
| Histopathology | H&E; LFB | |
| Optional tests | Tissue homogenate | Cytokines analysis |
| Peripheral blood flow cytometry | Flow cytometry assay | |
| Score | Symptoms | Description |
| 0 | Normal | Hold mouse by the base of the tail. Unaffected, mouse can "helicopter" tail. |
| 0.5 | Tail limpness | Hold mouse by the base of the tail. Some loss of tail tone. |
| 1 | Hold mouse by the base of the tail. Complete tail limpness, with no evidence of limb weakness. | |
| 1.5 | Limpness | No hind limb paralysis, but the hind limbs fail when ambulating on the lid metal grid. |
| 2 | Gait irregularities. Hold mouse by the base of the tail, the hind limbs are curled up. Or mouse's head is tilted, it can't keep balance with no hind limb paralysis. |
|
| 2.5 | Hind limbs paralysis | Partial paralysis of hind limbs, waddles upon ambulation, but does not drag limbs. Or mouse's head is tilted, and it keep fall down with no hind limb paralysis. |
| 3 | Partial paralysis of hind limbs as evidenced by dragging one limb upon ambulation. | |
| 3.5 | Forelimbs paralysis | Complete paralysis of both hind limbs, dragging body by forearms, still capable of moving around the cage. |
| 4 | Responsive but not moving/stationary, listless, rapid breathing (consult institutional veterinarian, consider euthanasia). | |
| 4.5 | Dead | Immobile and unresponsive, moribund (immediate euthanasia recommended). |
| 5 | - |
| Group | Onset (day) | Peak (day) | Incidence |
| G1: C57BL/6J |
12 | 16 | 5/5 |
| 12 | 16 | ||
| 14 | 17 | ||
| 12 | 16 | ||
| 12 | 16 | ||
| G2: C57BL/6N |
14 | 18 | 5/5 |
| 12 | 14 | ||
| 14 | 18 | ||
| 14 | 16 | ||
| 14 | 18 |
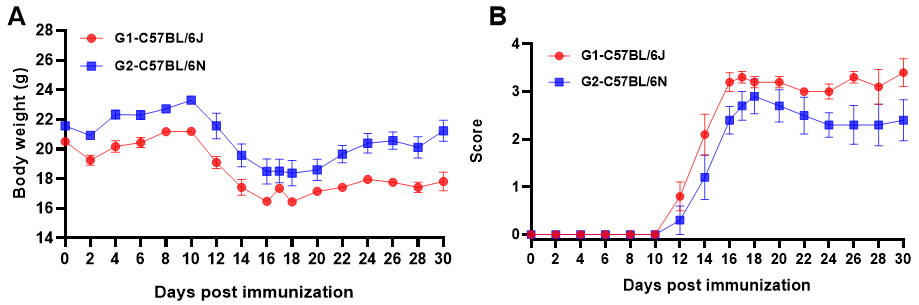
Fig1. MOG35-55 induced EAE. C57BL/6J and C57BL/6N (A, B) mice received MOG35-55 emulsion injection (s.c.) on neck and buttocks (C) the day 0. PTX (i.p.) were given 2 and 24 hour after MOG injection. Body weight and clinical score were recorded every two days. Values are expressed as mean ± SEM, n=5.
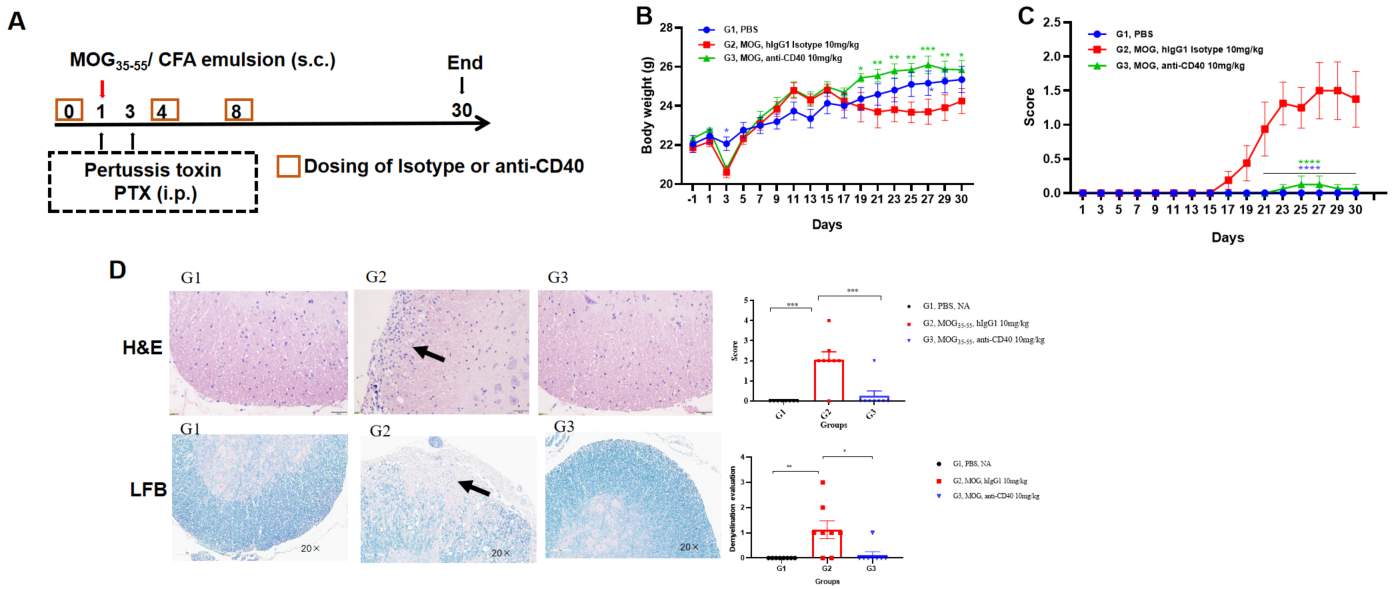
Effects of anti-CD40 on MOG35-55 induced EAE. Mice received MOG35-55 emulsion injection (s.c.) on flank (blue point) on day 0. PTX (i.p.) were given 2 and 48 hour after MOG injection. Isotype control or anti-CD40 were administered on day 0, 4 and 8. (A). Body weight (B) and clinical score (C) were recorded every two days. Immune cell Infiltration and demyelination were assessed by H&E and LFB staining of the spinal cord (D). Values are expressed as mean ± SEM, n=5. compared with G2, *p<0.05, **p<0.01, ***p<0.001 , **** p<0.0001.
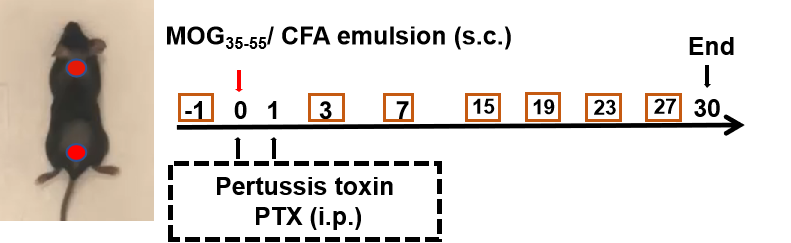
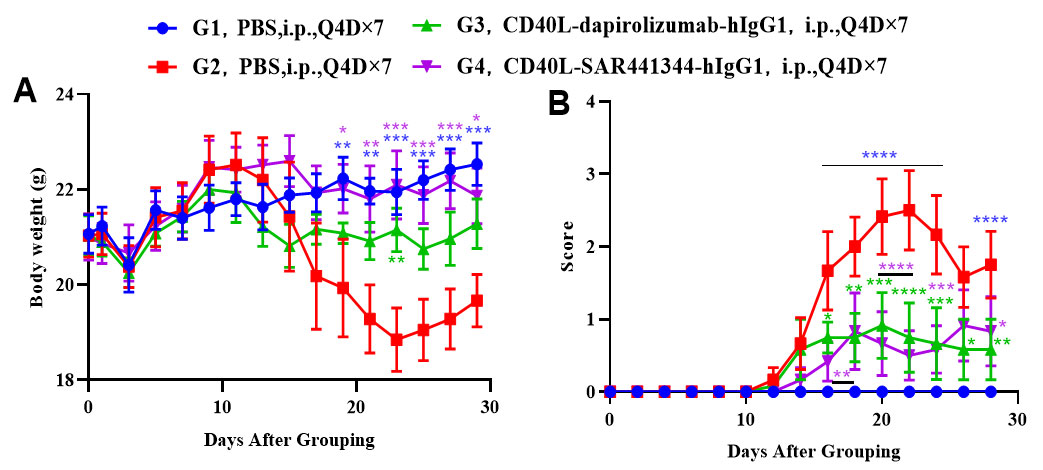
Effects of anti-CD40L on MOG35-55 induced EAE. Mice received MOG35-55 emulsion injection (s.c.) on neck and buttock (red point) on day 0. PTX (i.p.) were given 2 and 48 hour after MOG injection (G2-G4). Isotype control group(G3) or anti-CD40 antibodies group(G4) were administered BIW. Body weight (A) and clinical score (B) were recorded every two days. Values are expressed as mean ± SEM, compared with G2, *p<0.05, **p<0.01, *** p<0.001, **** p<0.0001.
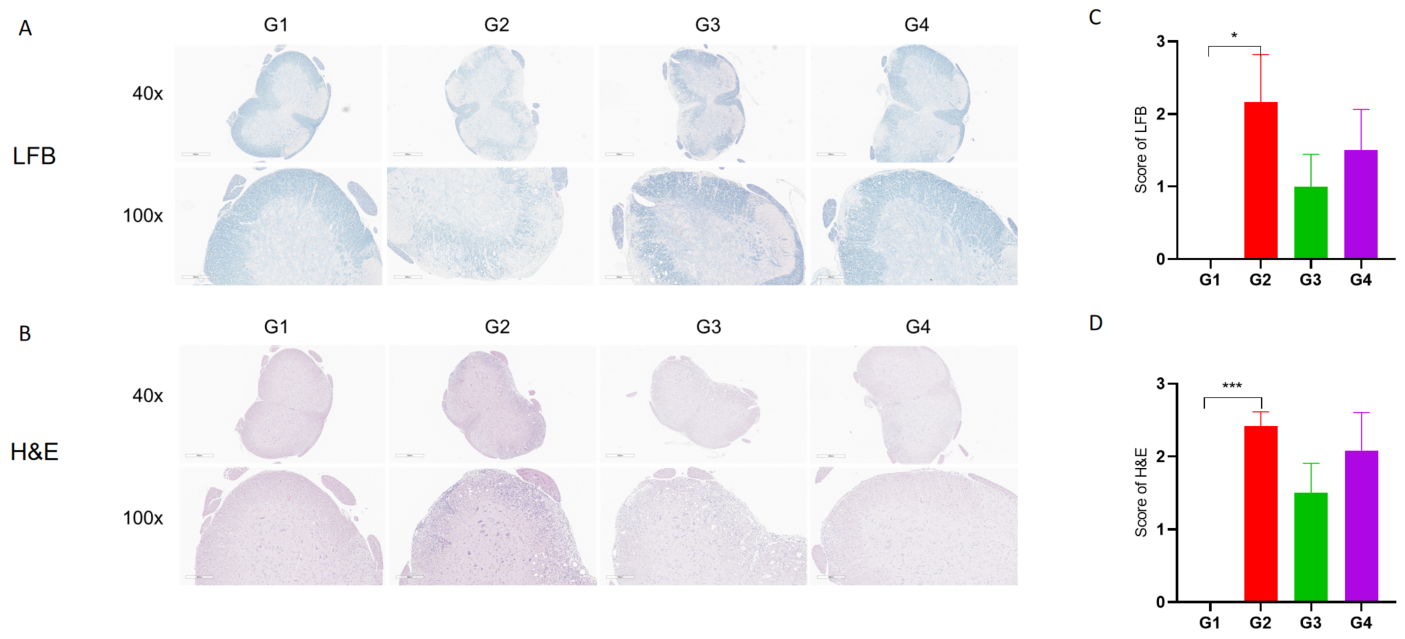
Anti-CD40L antibodies (analog, in-house) improves EAE clinical signs and controls inflammation and demyelination.
Spinal cords were removed from B-hCD40/hCD40L mice on day 30 and stained with Luxol fast blue (LFB) (A) or Hematoxylin and eosin (H&E) (B). Representative sections are shown. The score of inflammatory cells and demyelination of spinal cord (C&D). Values are expressed as mean ± SEM, compared with G2, *p<0.05, **p<0.01, *** p<0.001.
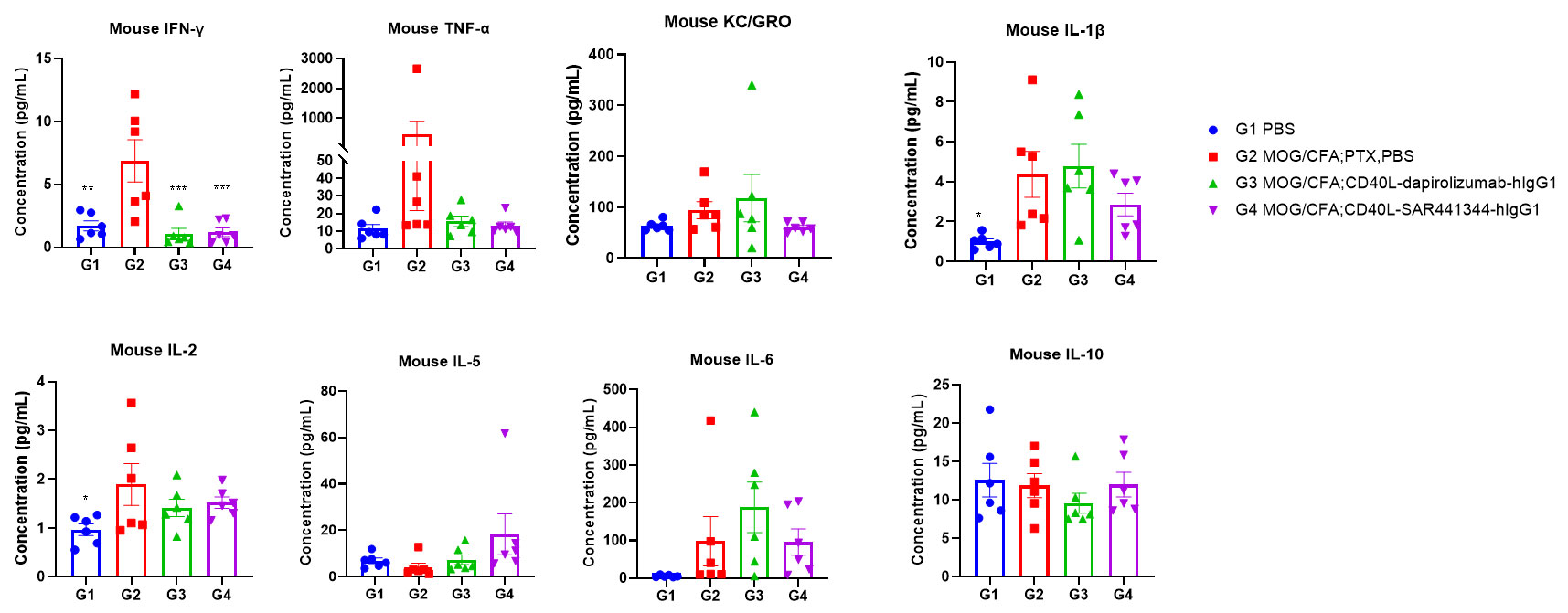
Effects of anti-CD40L on MOG35-55 induced EAE. Mice received MOG35-55 emulsion injection (s.c.) on neck and buttock (red point) on day 0. PTX (i.p.) were given 2 and 48 hour after MOG injection. Isotype control or anti-CD40 antibodies were administered BIW. Serum were collected at study endpoint and the cytokine levels were assessed. Values are expressed as mean ± SEM, n=6, compared with G2, *p<0.05, **p<0.01, *** p<0.001, **** p<0.0001.