

Based on various in vivo models and in vitro platform, we have established a state-of-the-art ADC evaluation platform to study the efficacy, mechanism of action and the functional effects of ADCs as well as the combination therapies.
on this page
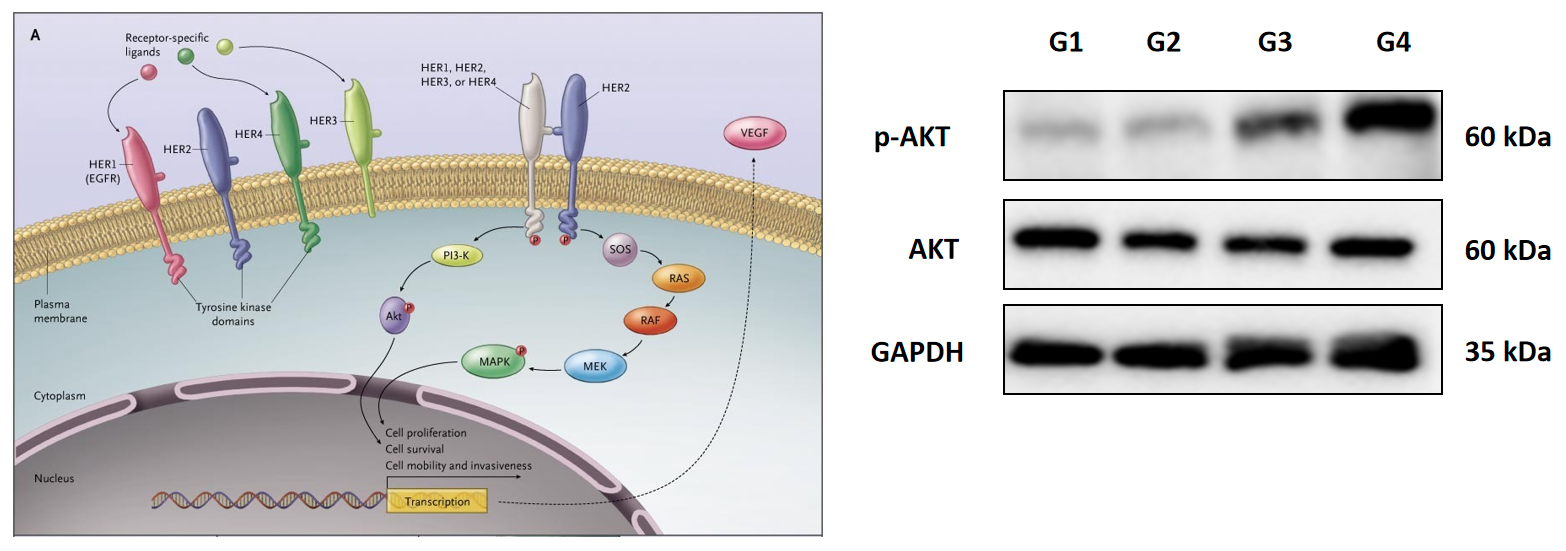
Antibody–drug conjugates (ADCs), one of novel therapeutic biologics, are composed of a monoclonal antibody that recognizes a specific tumor antigen, an extremely potent cytotoxic drug known as payload, and a linker connecting the payload to the antibody, aiming to take advantage of the specificity of monoclonal antibodies to deliver potent cytotoxic drugs selectively to antigen-expressing tumor cells. Recently, ADCs have been considered as an important means of future disease treatment and the global upsurge of that has been risen up.
Based on robust in vivo and in vitro platform, we have established a state-of-the-art ADC evaluation platform to study the efficacy, mechanism of action and the functional effects of ADCs as well as the combination strategy of ADCs and other anti-cancer therapy for preclinical research of both McAb-ADC and BsAb-ADC.
Our flow cytometry and cell-based platforms support a wide range of in vitro studies in a fast, reliable, and reproducible manner, including the evaluation of antibody affinity and binding, internalization processes, payload efficacy (apoptosis and cell cycle), and the efficacy of antibody fragments (ADCC, CDC, ADCP). For in vivo ADC assessments, our experienced team and extensive collection of CDX models facilitate efficient efficacy evaluations of ADCs and combination treatments. Additionally, we have developed a series of TAA target-humanized mouse models that can be used for toxicity studies. We also specialize in generating customized antigen-specific overexpression or knock-in cell lines, tailored for ADC development that specifically targets those antigens.
| Projects | Experiments | Methods | ||
| Binding Assays | Target Antigen Binding (Same Family) | Binding activity detection | SPR/BLI | OK |
| Target Antigen Cross-Binding(Human, Mouse, Monkey) | Binding activity detection | SPR/BLI | OK | |
| FcRn Binding | Binding activity detection | SPR/BLI | OK | |
| FcγR Receptor Family Binding | Binding activity detection | SPR/BLI | OK | |
| C1q Binding | Binding activity detection | ELISA | OK | |
| Functional Studies (in vitro) |
Cell screening | Protein expression detection | Flow cytometry, IHC | OK |
| Antibody binding | Antibody concentration titration | Flow cytometry | OK | |
| Cytotoxicity | Proliferation inhibition | CTG, Prestoblue, IncuCyte | OK | |
| Bystander effect | Flow cytometry, Incucyte | OK | ||
| Mechanistic research | Antibody endocytosis | Flow cytometry, Incucyte, Confocal | OK | |
| Apoptosis | Flow cytometry, Incucyte | OK | ||
| Cell Cycle | Flow cytometry | OK | ||
| ADCC, CDC, ADCP | Flow cytometry, LDH, CTG, Prestoblue | OK | ||
| Lysosome Co-localization | confocal | OK | ||
| Ligand Blocking | Flow cytometry | OK | ||
| Signaling pathways | Western Blot, MSD … | OK | ||
| Pharmacokinetics (ex vivo) |
Stability | Test stability of ADC in plasma (Human, Mouse, Monkey) | LC-MS | OK |
| PK Profile | Serum concentration monitoring | ELISA, MSD | OK | |
| Efficacy Studies (in vivo) |
Anti-tumor efficacy | CDX model | Tumor volume | OK |
| PDX model | Tumor volume | OK | ||
| Orthotopic model | Bioluminescence | OK | ||
| Bystander effect | Bioluminescence | OK | ||
| Tissue distribution | ELISA, MSD | Developing |
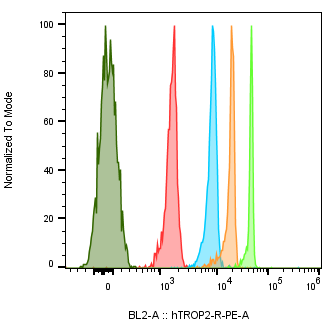
Trop2 expression in tumor cell lines by absolute quantification
| Cell Line Name | Trop2 Expression level (/cell) |
| NCI-N87 | 667,835 |
| A431 | 3,816,803 |
| MCF-7 | 445,552 |
| NCI-H292 | 1,375,075 |
| NCI-H520 | 822 |
| NCI-H1975 | 401,381 |
| HCC827 | 821,464 |
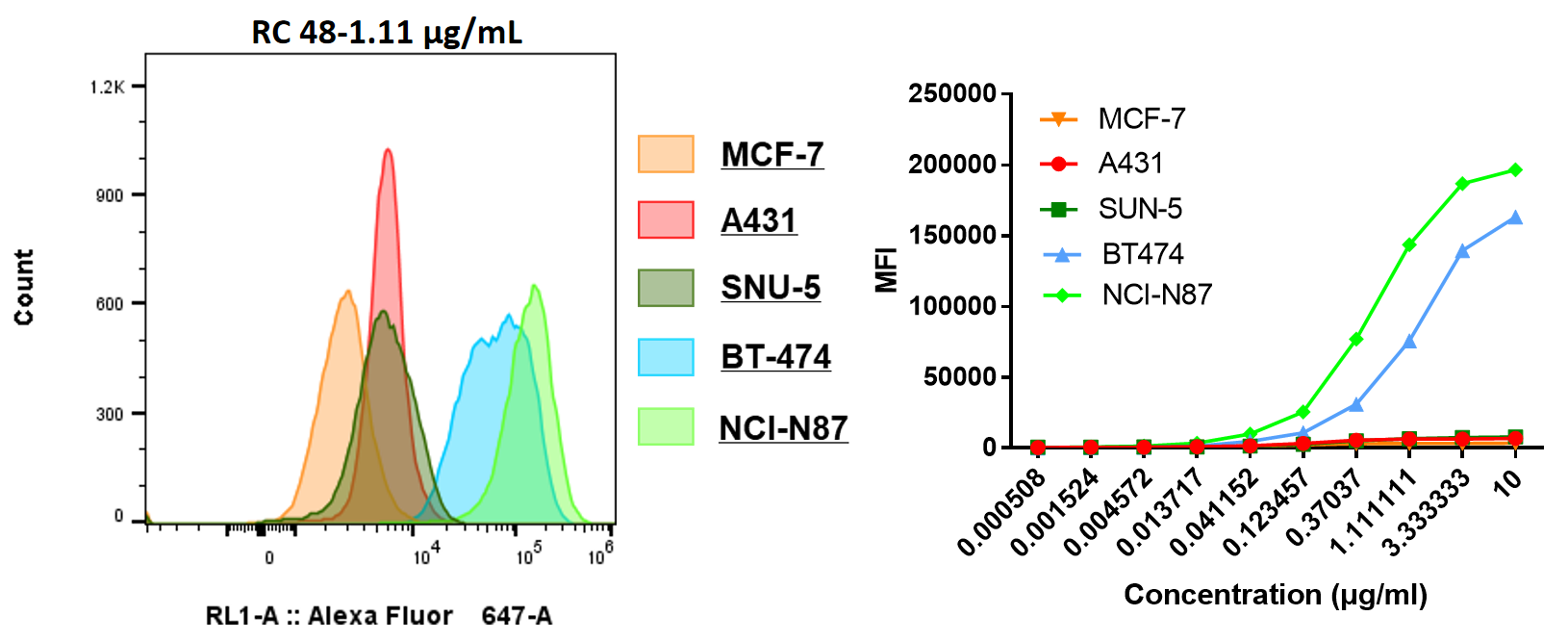
| EC50 (μg/mL) | |
| NCI-N87 | 0.511 |
| MCF-7 | - |
| BT474 | 1.042 |
| SNU-5 | - |
| A431 | - |
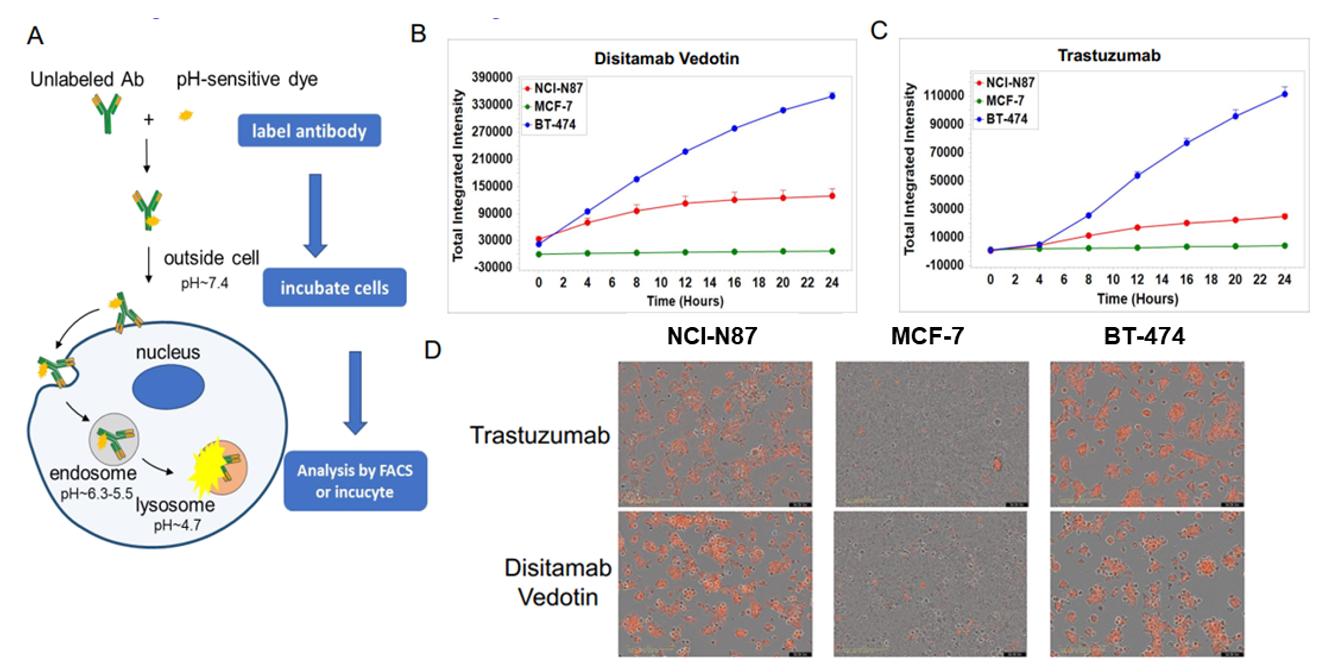
In vitro internalization assay of Disitamab Vedotin and trastuzumab. A: The schematic diagram of antibody internalization assay. NCI-N87, MCF-7, and BT-474 cells were incubated with pH-sensitive-dye-labeled disitamab vedotin(RC-48) or trastuzumab for 24hr and observed continuously with Incucyte. B-C: The ordinate indicates the quantity of intracellular antibodies. D: The orange signals in the photos indicate the internalized antibodies. Both disitamab vedotin(RC-48) and trastuzumab were internalized into NCI-N87 and BT-474 cells in a time-dependent manner, whereas not in MCF-7 cells.
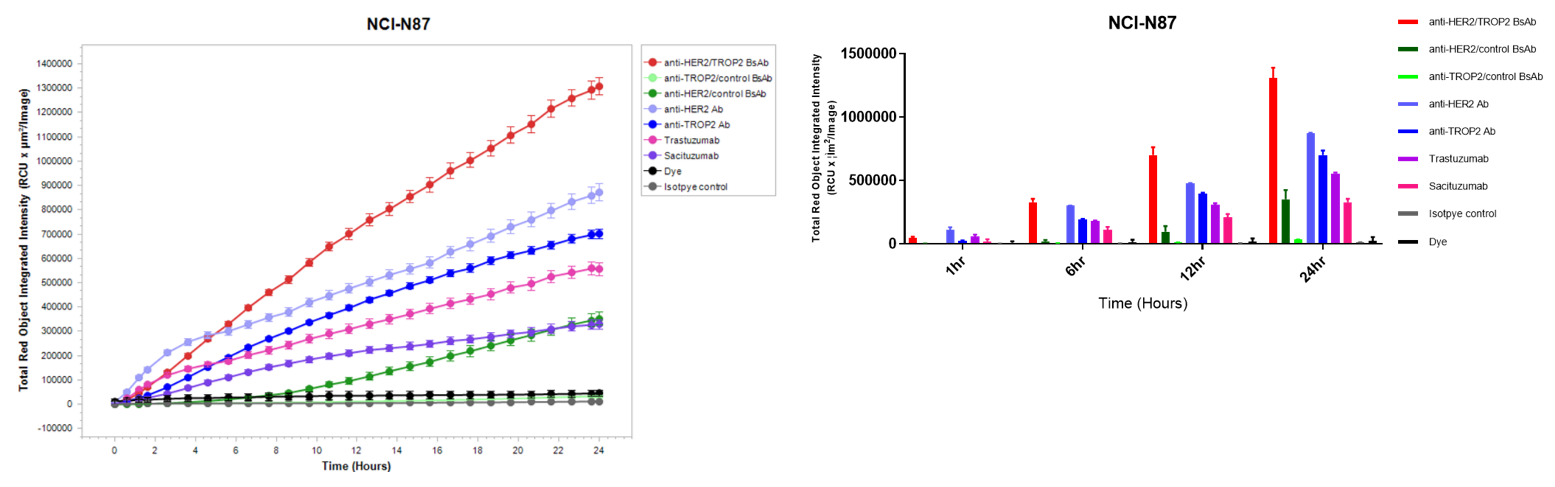
| gene_name | FPKM NCI_N87 |
| HER2 | 1444 |
| TROP2 | 761 |
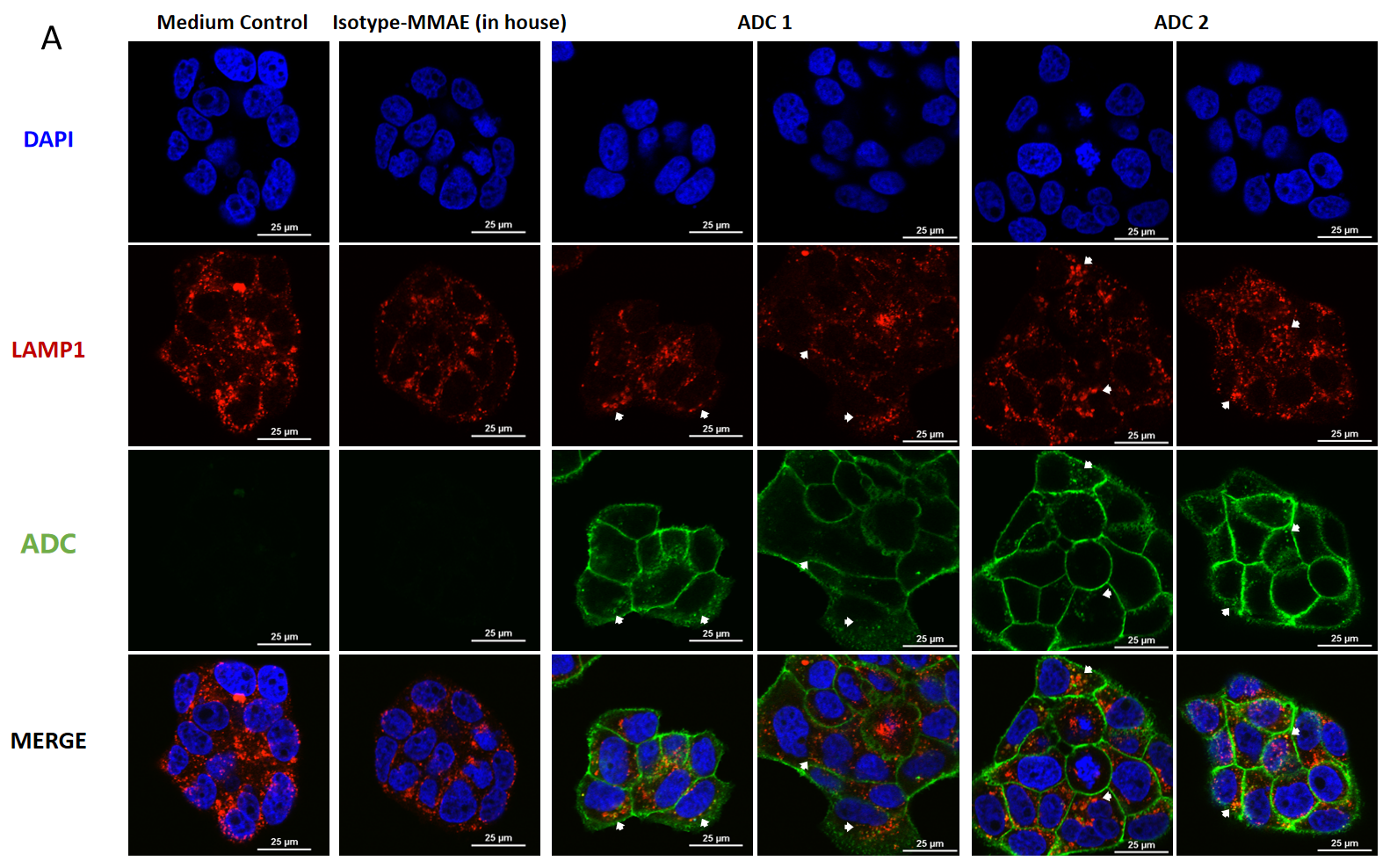
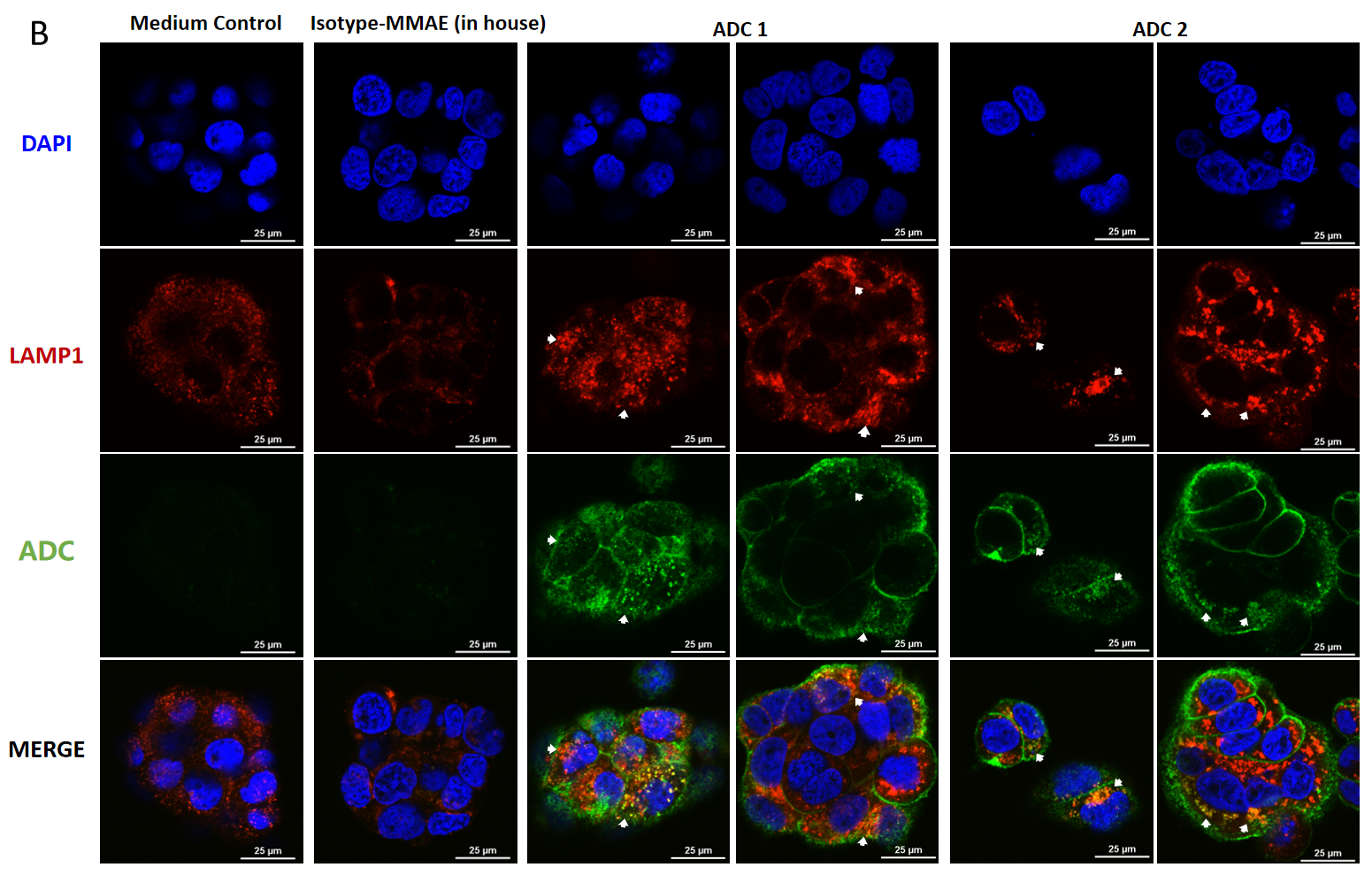
Confocal microscopy images of BT474 (Her2+ and Trop2+) cells showing internalization and lysosomal trafficking of MMAE-conjugated ADC.
BT474 cells were treated with ADC for (A) 4hr and (B) 24hr (10 μg/mL each ADC), and stained by FITC-conjugated goat anti-human IgG antibody (green); lysosomes were stained by LAMP1 mouse monoclonal antibody and Cy3-conjugated goat anti-mouse IgG secondary antibody (red); nuclei were stained by DAPI (blue). Scale bars, 25 μm.
| MDA-MB-468: BT474 | ||
| 0:1 | 1:0 | 1:1 |
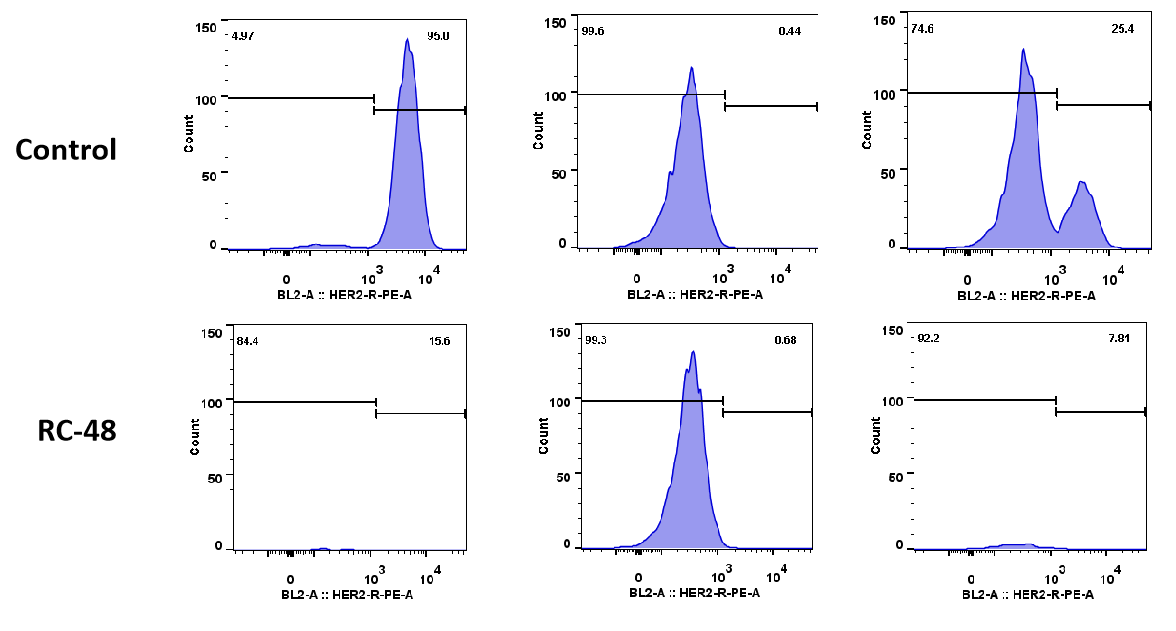
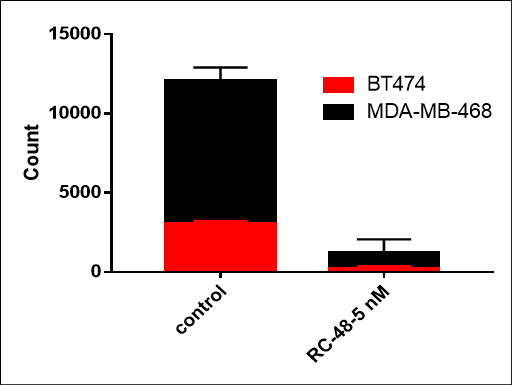
MDA-MB-468 cells ( Her2-) and BT474 (Her2+) were co-cultured overnight in different proportion (respectively 0:1, 1:0, 1:1). After treated with RC48 or vehicle for 5 days, cell number and ratio of HER2-positive and HER2-negative cells were determined by flow cytometer. RC48 showed great tumoricidal effects for BT474 cells, while almost no cytotoxicity for MDA-MB-468 cells. Importantly, RC48 could also kill MDA-MB-468 cells when cocultured with BT474 cells.
| B-CAG-luc-GFP KI, EGFR KO BxPC-3: BxPC-3 | ||
| 1:1 | 1:0 | 0:1 |
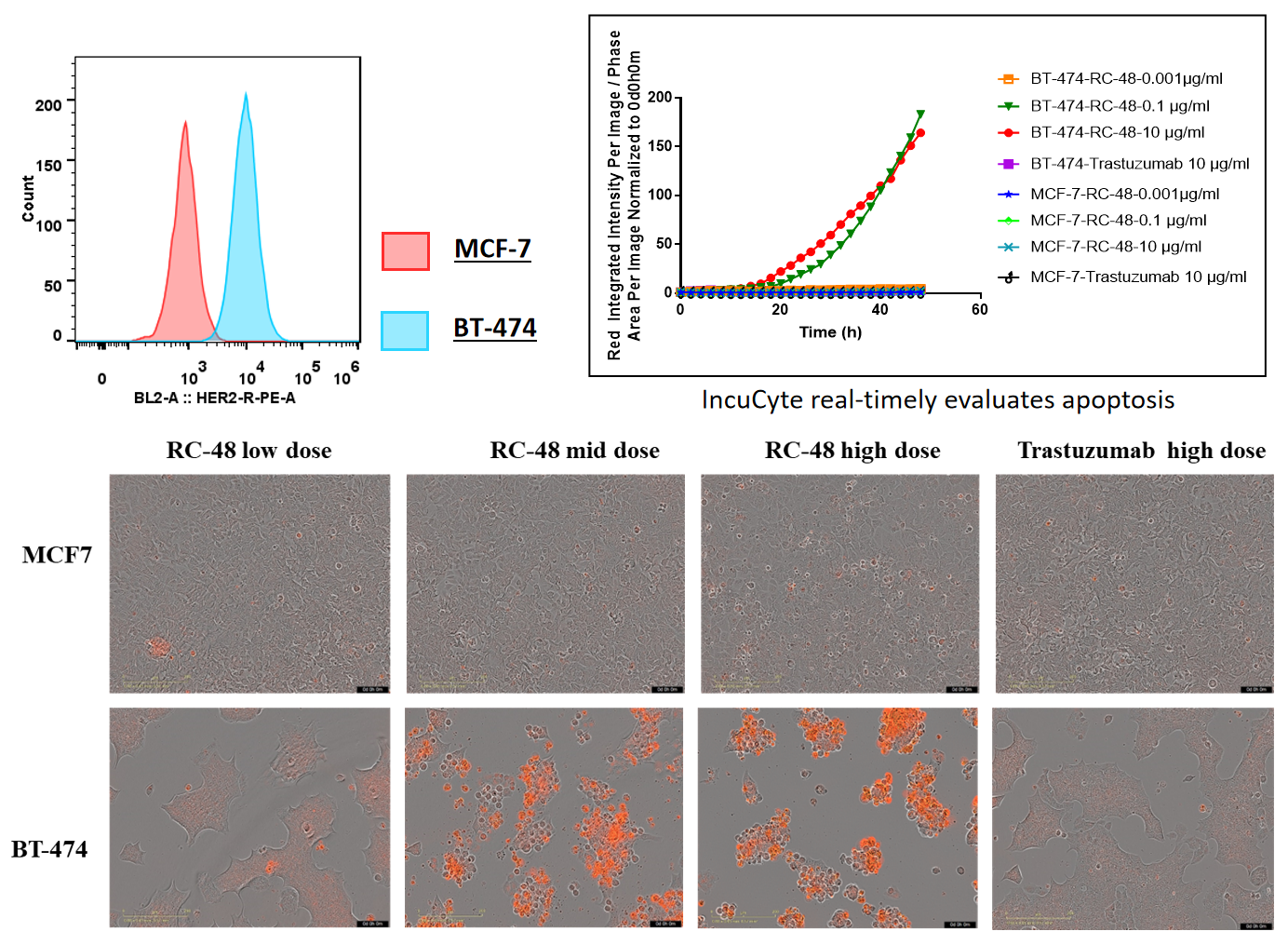
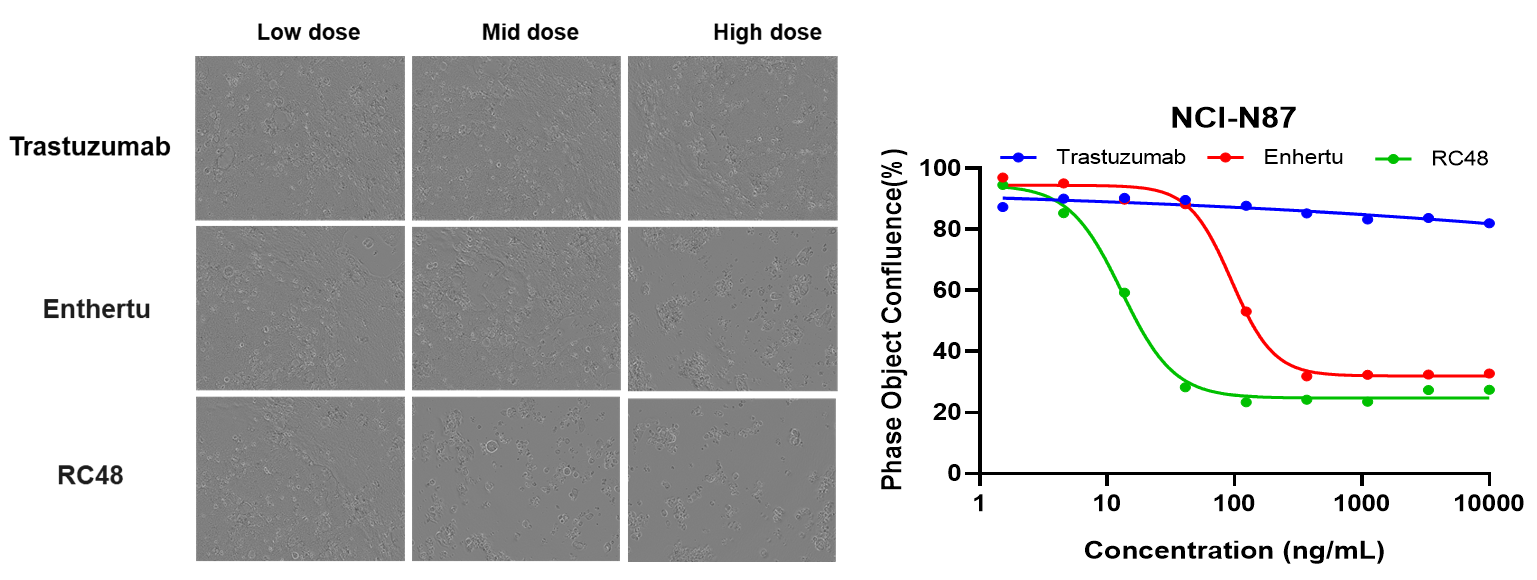
BT-474 (Her2+) and MCF7 (Her2-) cells were treated with different concentrations of trastuzumab or RC48 for 72h. Apoptosis was detected with Sartorius IncuCyte Caspase-3/7 red Apoptosis Regent. Red signals indicate the activated caspase 3/7. RC48(mid or high dose) significantly promoted BT-474 apoptosis, while trastuzumab not. Similarly, apoptosis was not observed on treated MCF7 cells.
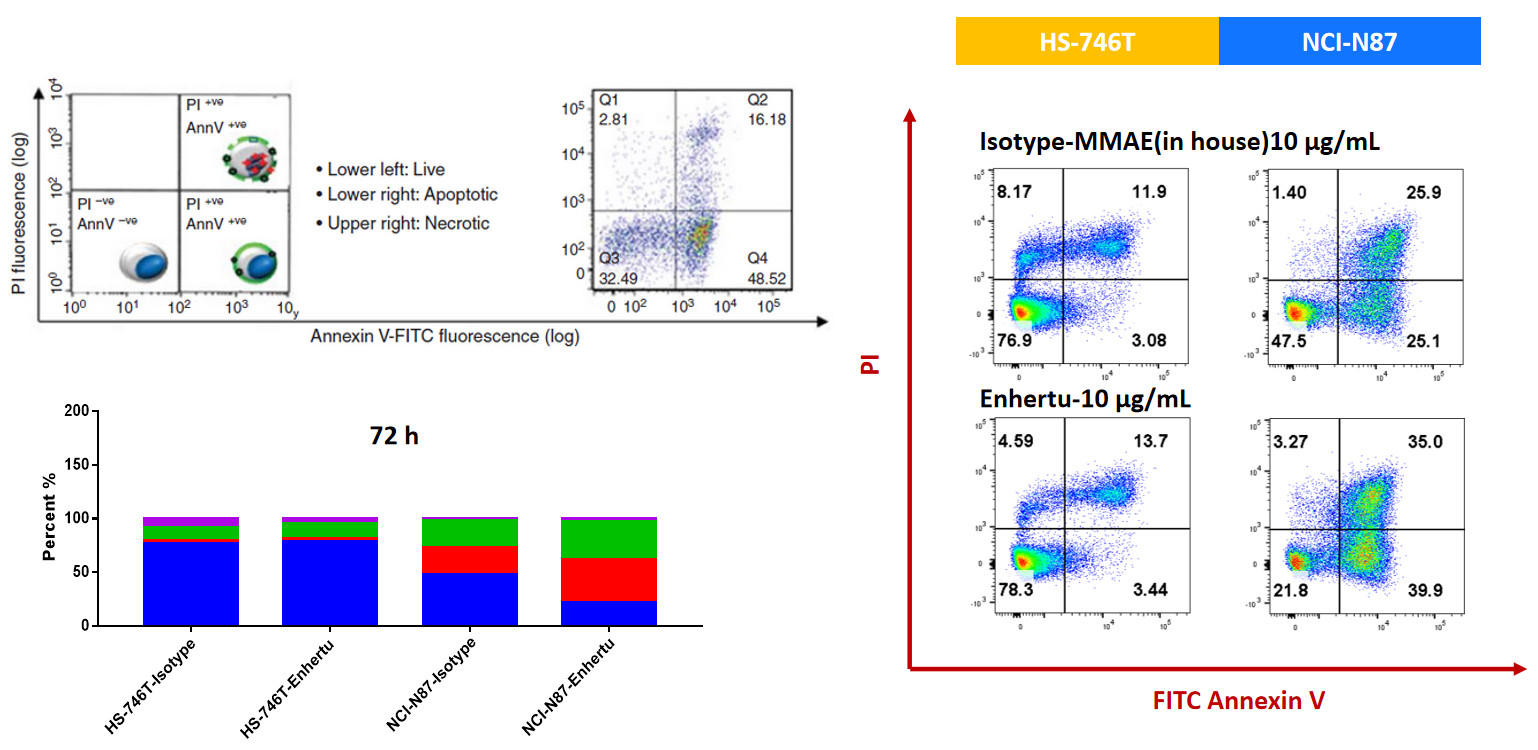
NCI-N87 (Her2+) and HS-746T (Her2-) cells were treated with ISO-MMAE and Enhertu for 72h. Apoptosis was detected with Annexin V/PI kit by flow cytometry. For Her2 high-expressed NCI-N87, Enhertu could induce significantly apoptosis.
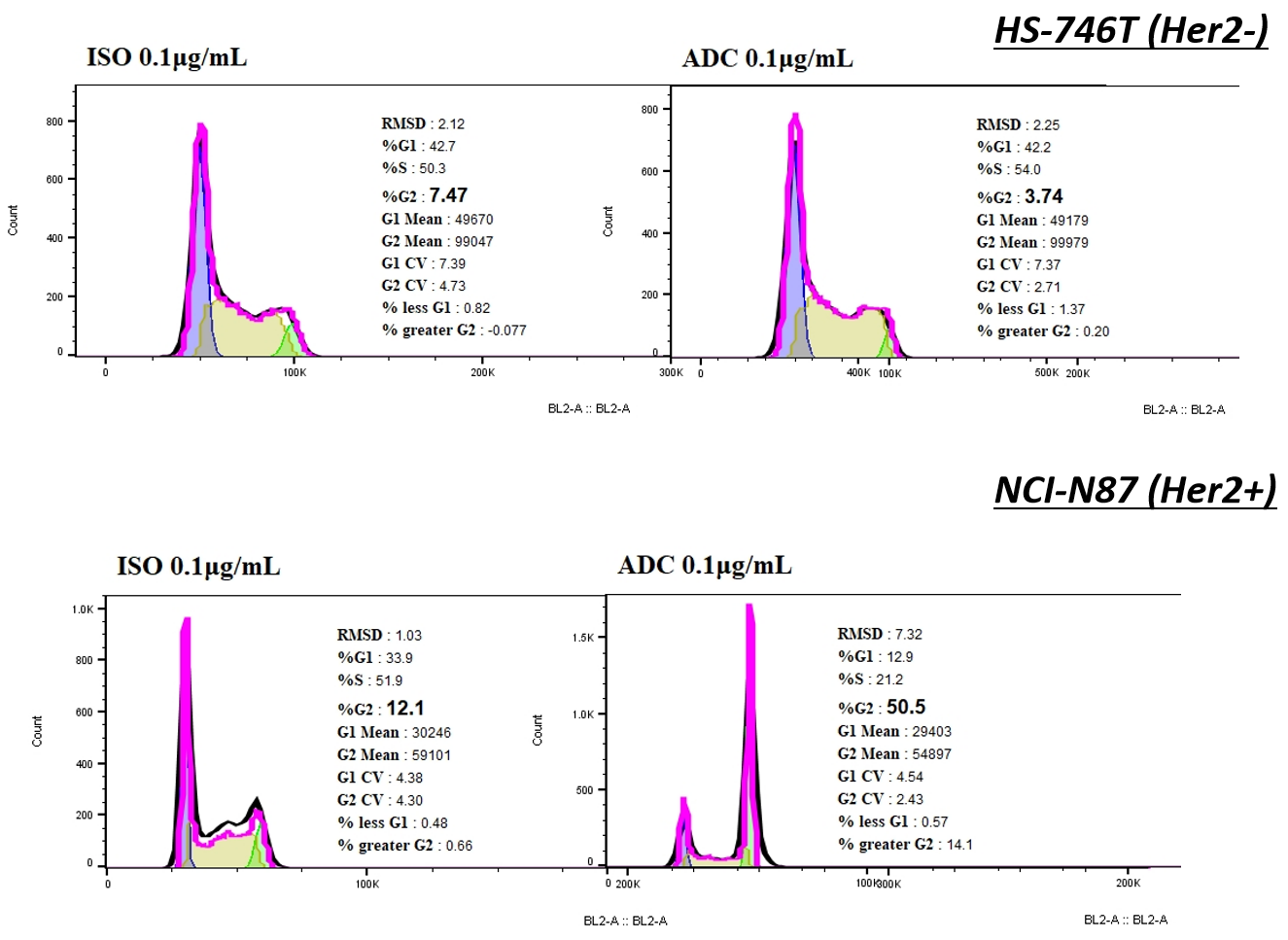
Compared with ISO-MMAE, Her2-MMAE could elicit cell-cycle arrest on NCI-N87, accompanied with a sharp increase proportion of cells in G2/M, while it was not observed on HS-746.
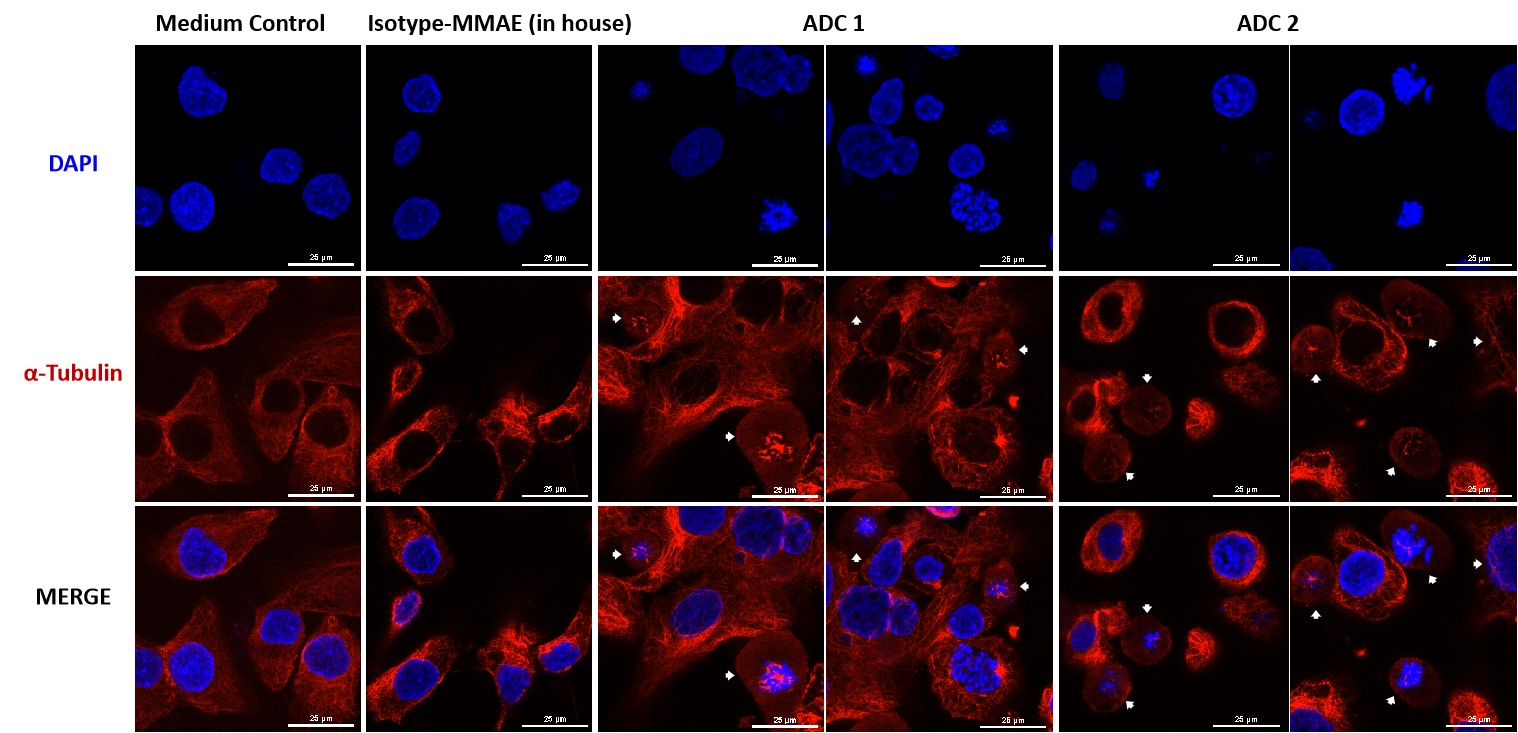
The MMAE-conjugated ADC disrupts the intracellular microtubule network observed by confocal microscopy.
SKOV-3 (Her2+ and Trop2+) cells were treated with MMAE-conjugated ADC for 24h (10 μg/mL each ADC). Microtubules stained by an PE-conjugated α-Tubulin mouse monoclonal antibody (red) and nuclei were stained by DAPI (blue). Scale bars, 25 μm.
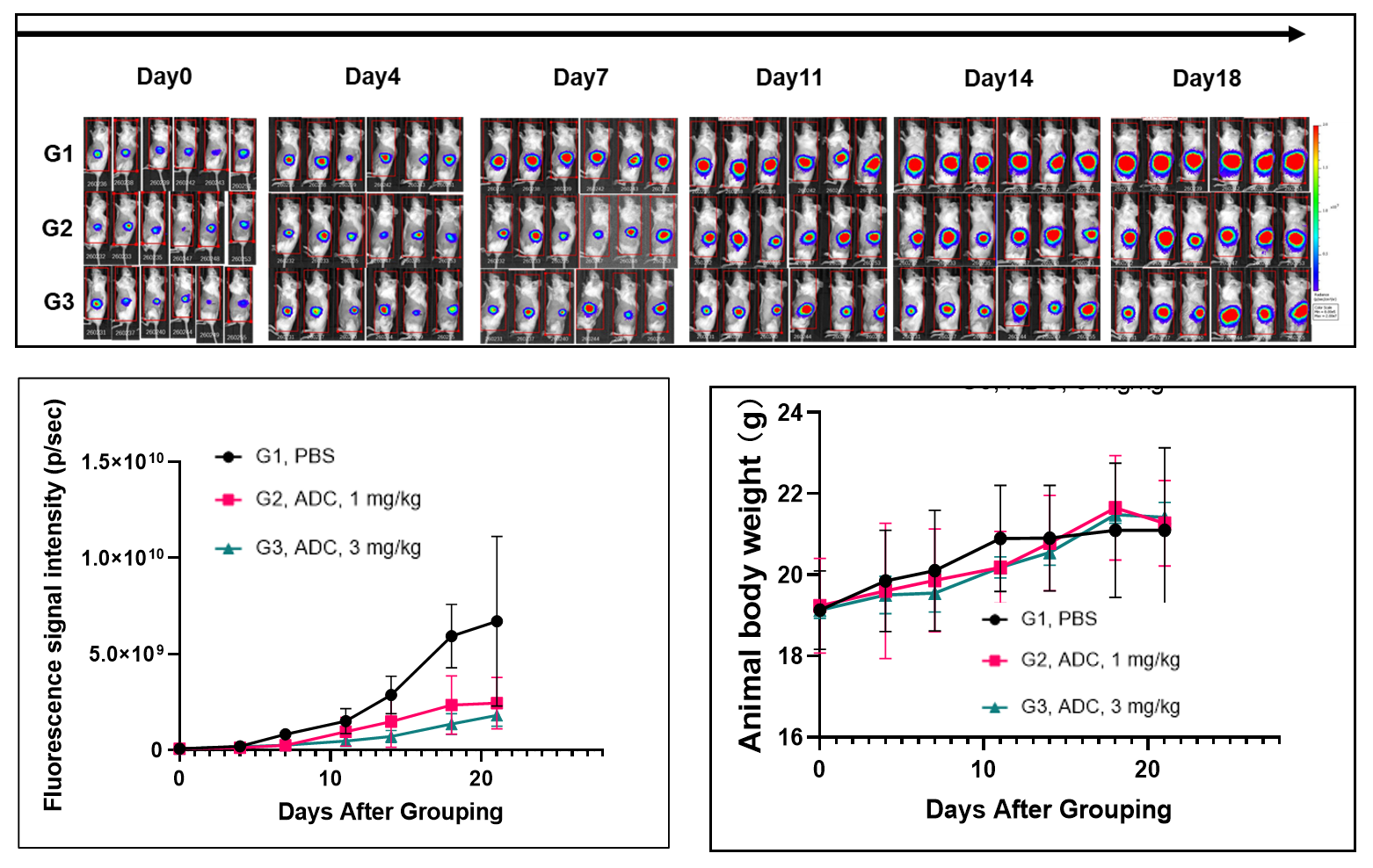
Pancreatic cancer cell line B-luc MIA Paca-2 orthotopic inoculation, tumor growth was significantly inhibited after Her2-ADC treatment.
Click Here to Check our Orthotopic CDX Model List
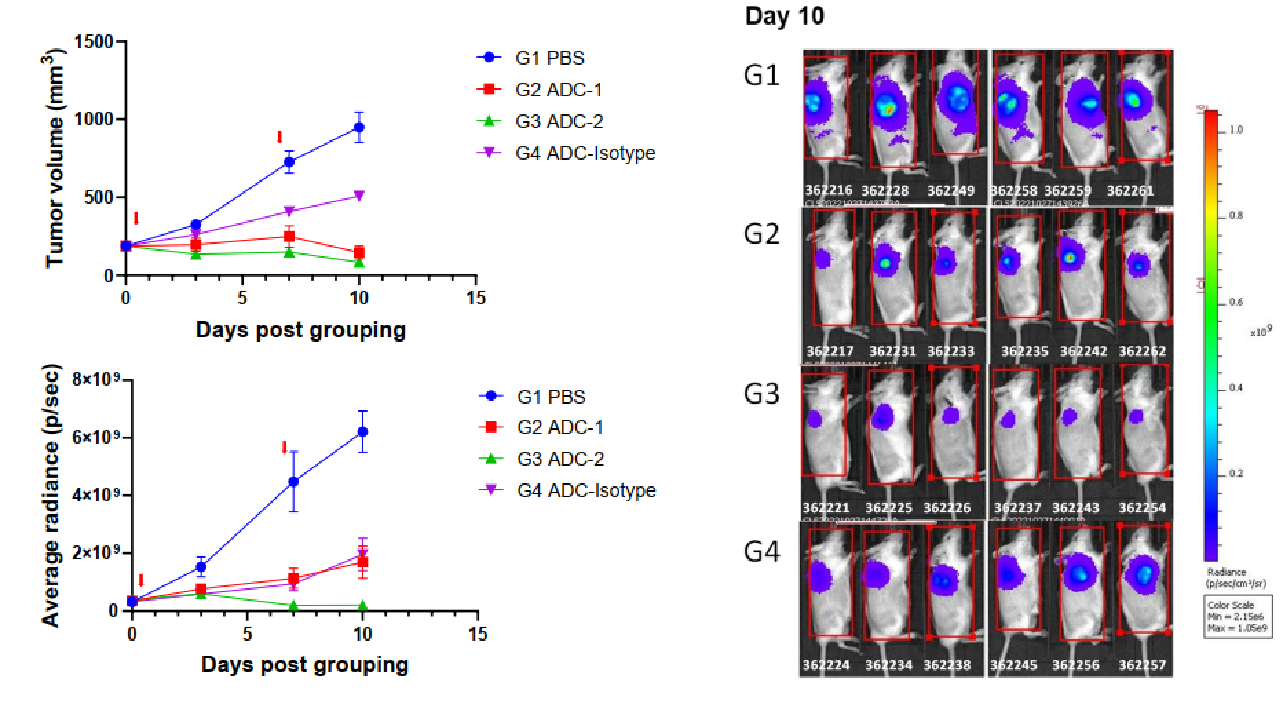
Bystander killing in co-inoculated conditions in vivo. Seven days after inoculation of a mixture of both NCI-H1975(target positive) and B-luc Daudi (target negative) cells, mice were i.v. given antibody–drug conjugates (day 0, day7). Luciferase activity was detected by in vivo imager after i.v. administration of substrate. (Left top) Estimated tumor volume; (Left bottom) Luciferase activity; (Right) Imaging data of luciferase activity on day 10, (n = 6). As shown in the figure, ADC2 could also significantly inhibit B-luc Daudi growth, while ADC1 could not.
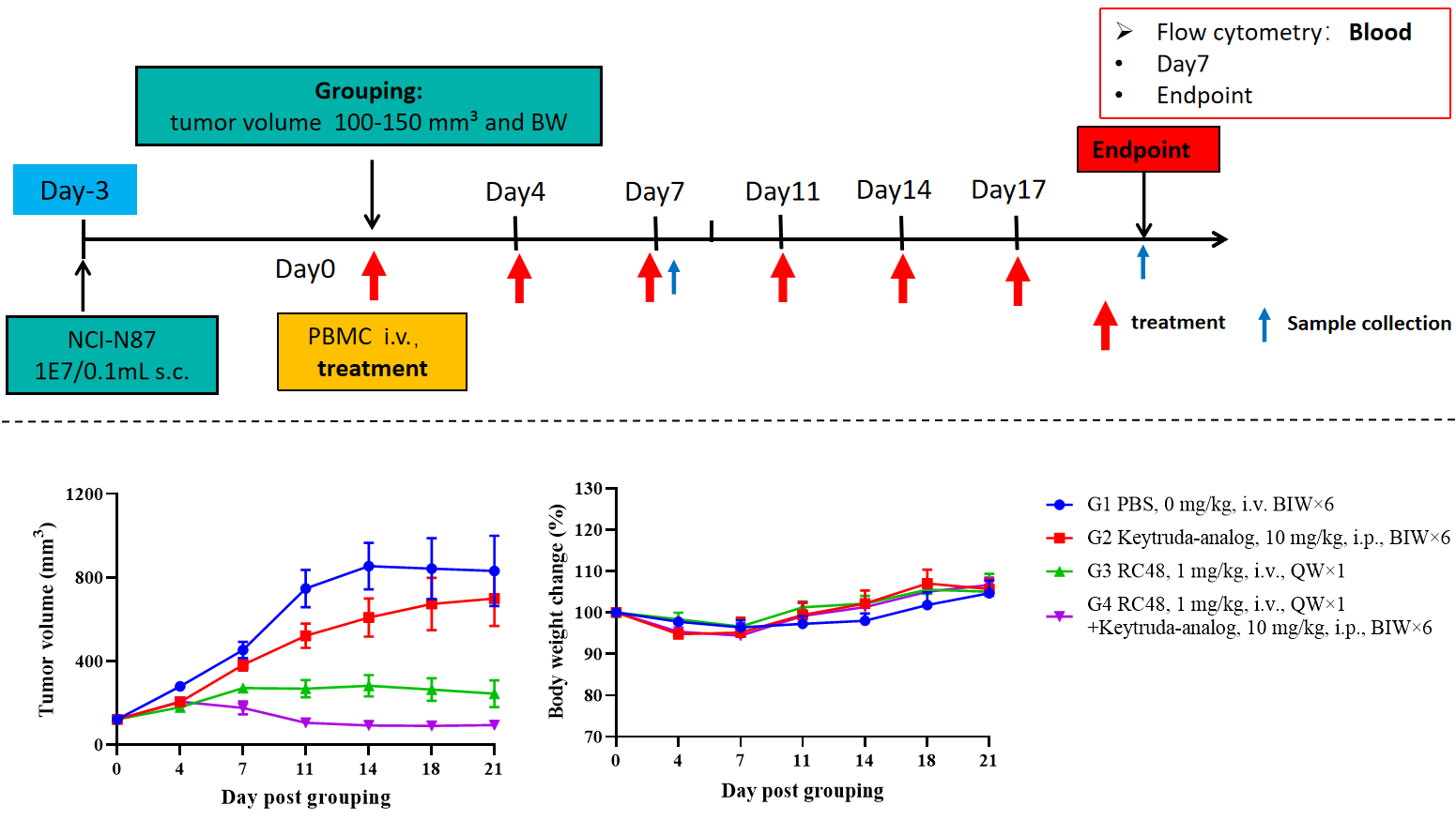
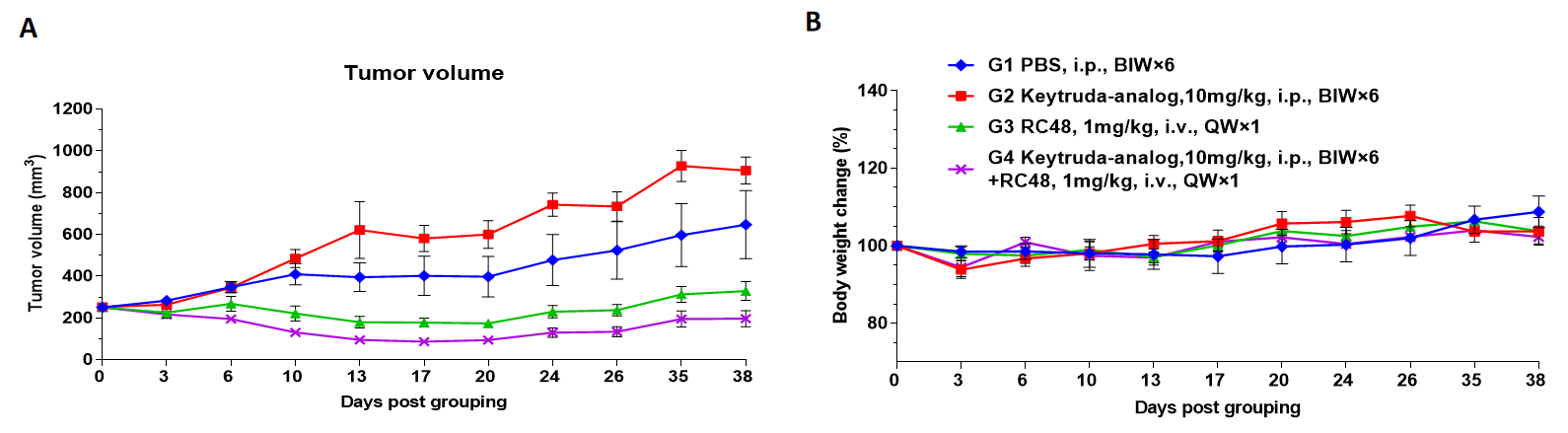
B-NDG hIL15 mice reconstituted with CD34+ cells were used for drug efficacy studies.
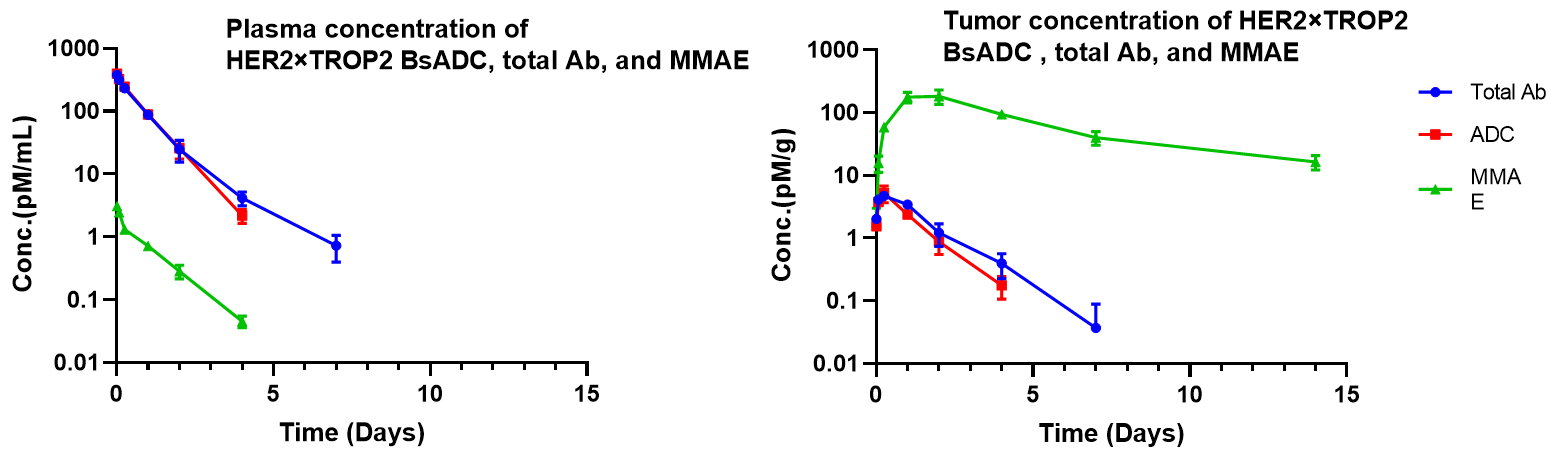
Pharmacokinetic analysis of HER2×TROP2 BsADC in NCI-H1975 xenograft model. After a single dose of 3 mg/kg of HER2×TROP2 BsADC , the payload MMAE was found to accumulate in the tumor but was present at low levels in the plasma. This suggests that HER2×TROP2 BsADC has reached an efficient tumor-targeted delivery of MMAE and may have better antitumor activity.