

Biocytogen uses wild-type C57BL/6 mice and humanized mice to create atopic dermatitis (AD) models via oxazolone or MC903 for preclinical drug evaluation, complete with full datasets.
on this page
Atopic dermatitis (AD) is a chronic skin disease that presents with itching, erythema and squamous lesions, and can be associated with cardiovascular disease, mental disorders (depression, anxiety, sleep disorders, etc.) and other diseases.
Atopic dermatitis mouse models:
Biocytogen uses wild-type C57BL/6 mice and humanized mice to create a series of atopic dermatitis (AD) models via oxazolone or MC903. These models are suitable and reliable for evaluating the preclinical efficacy of drug candidates, supporting AD therapeutic development.
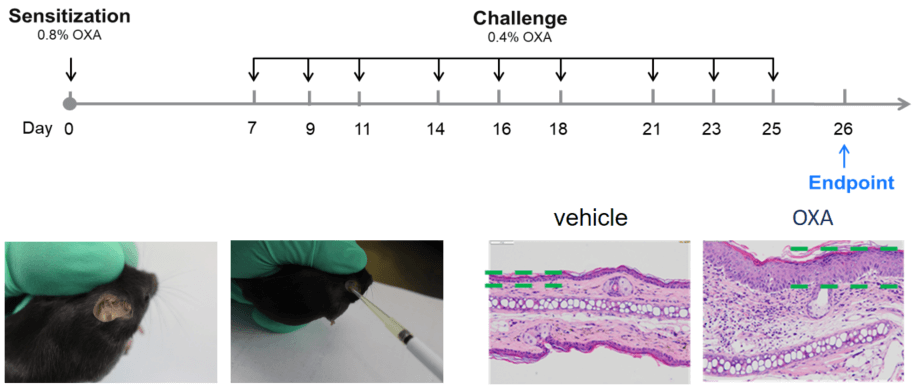
| Readouts | ||
| Included tests | Clinical scores | Ear thickness |
| Ear histopathology (H&E) | Immune infiltration | |
| Epidermal thickness | ||
| Histology scores | ||
| Optional tests | Molecular levels | Serum total IgE |
| Protein level (Elisa or Luminex) | ||
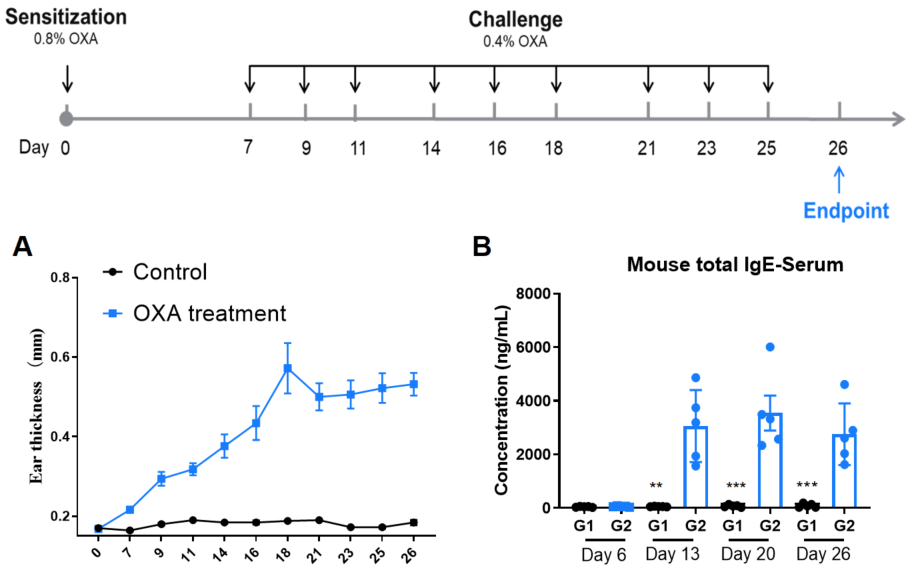
Establishment of AD model of C57BL/6 mice. A. Ear thickness change during treatment. B. Mouse total IgE in serum of each group on Day 6, 13, 20, 26. (A. Two-way ANOVA; B. One-way ANOVA; ** p< 0.01, *** p< 0.001).
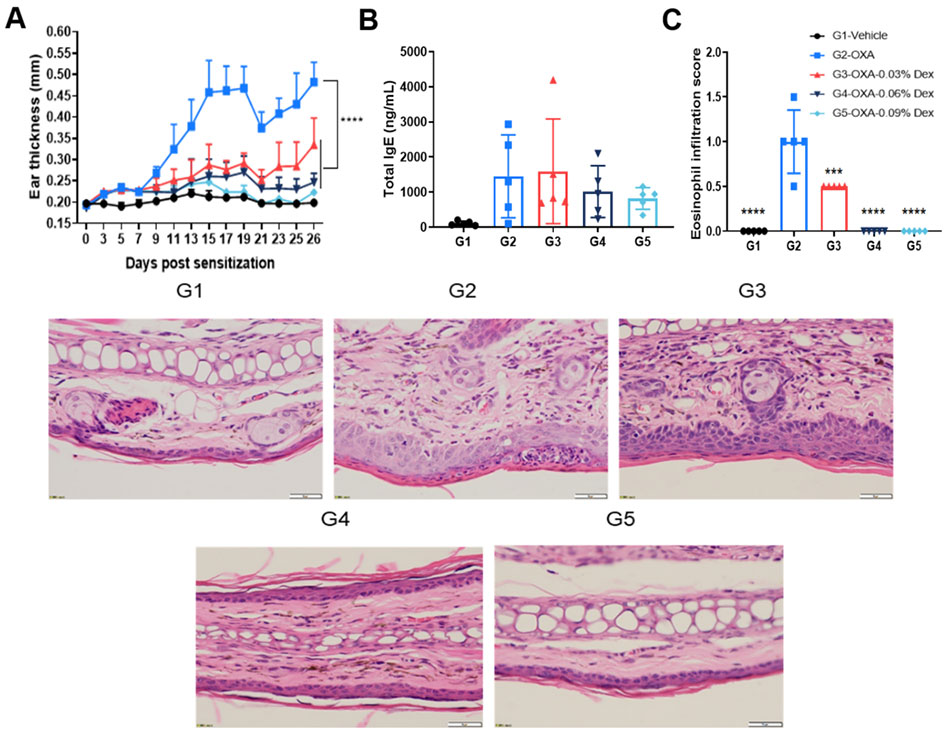
Efficacy evaluation of dexamethasone in AD model of C57BL/6 mice. A. Ear thickness change during treatment. B. Mouse total IgE in serum. C. Eosinophil infiltration score of ear histopathology. (A-B. Two-way ANOVA; C-D. One-way ANOVA; *** p< 0.001, **** p< 0.0001).
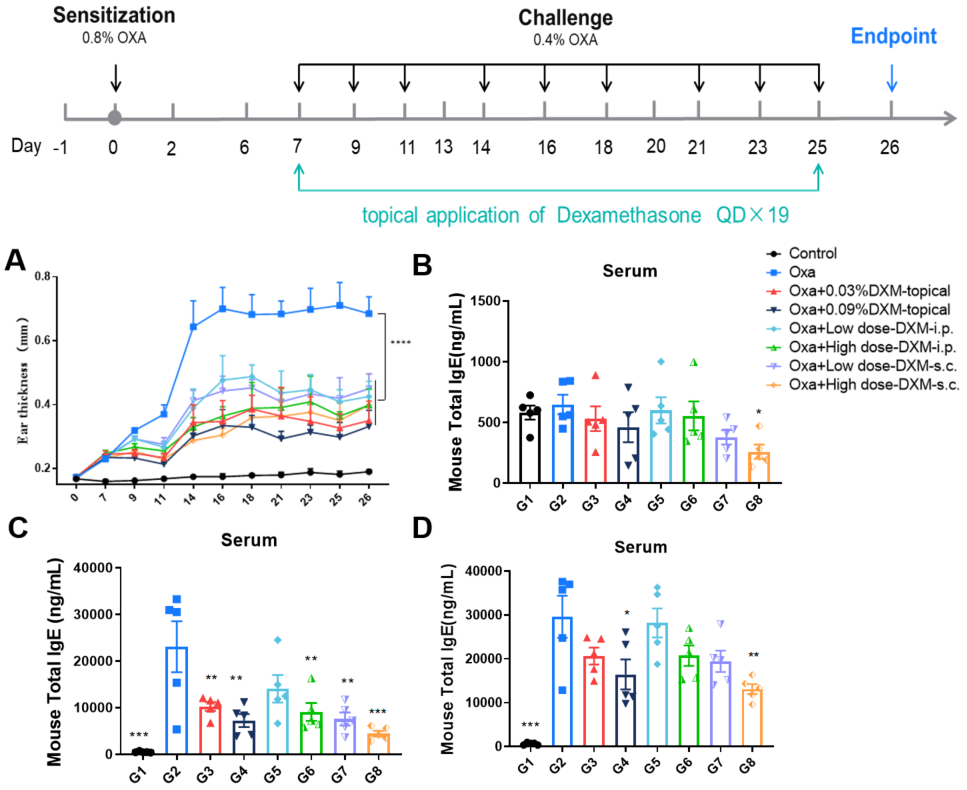
Efficacy evaluation of dexamethasone in AD model of BALB/c mice. A. Ear thickness change during treatment. B-D. Mouse total IgE in in serum on Day 7, 17, 26. (A. Two-way ANOVA; B-D. One-way ANOVA; *p<0.05, **p<0.01, *** p< 0.001).
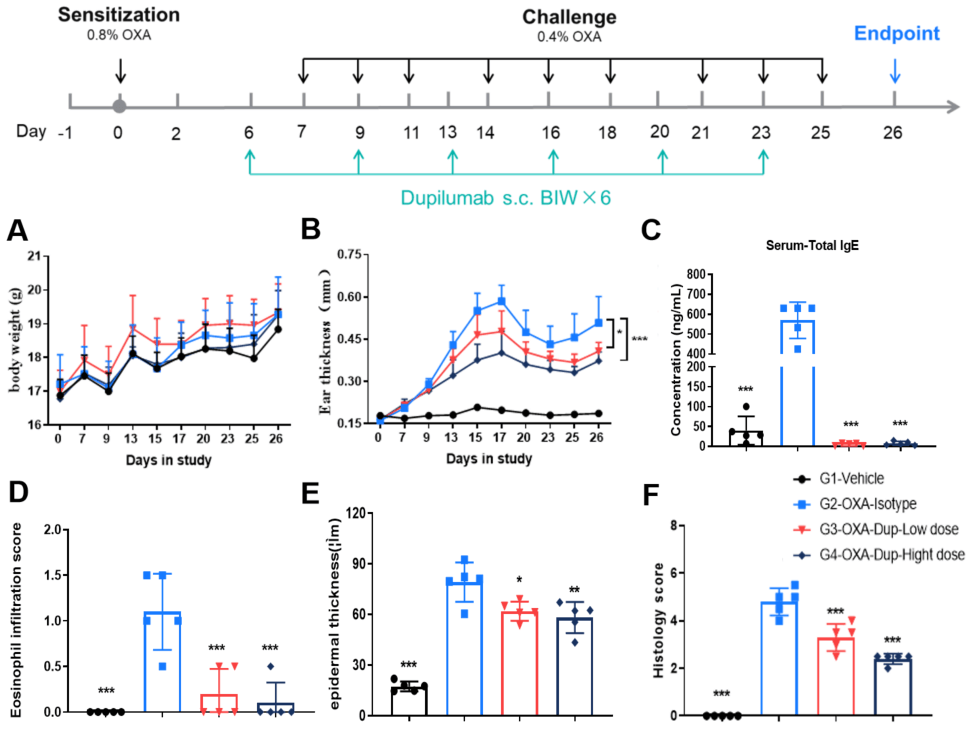
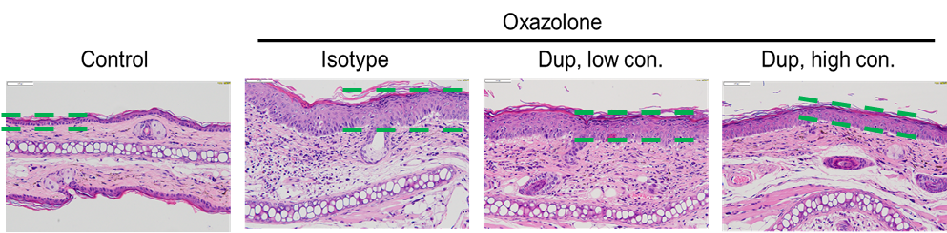
Efficacy evaluation on B-hIL4/hIL4RA AD models for dupilumab. A. Body weight change during treatment. B. Ear thickness change during treatment. C. Mouse total IgE in serum. D. Eosinophil infiltration score. E. Epidermal thickness. F. Histology score. (A-B. Two-way ANOVA; C-F. One-way ANOVA; *p<0.05, **p<0.01, *** p< 0.001).
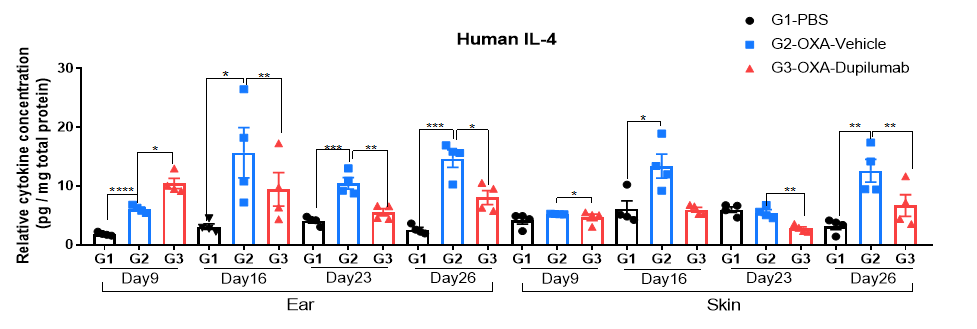
Protein expression analysis in OXA-induced AD model of B-hIL4/hIL4RA mice by ELISA. Ear samples of modeling area were collected on day 9, 16, 26 and 26 and analyzed ELISA. Tissue sample homogenate supernatants were loaded for ELISA detection, and the results were standardized by total protein concentration of the corresponding sample.
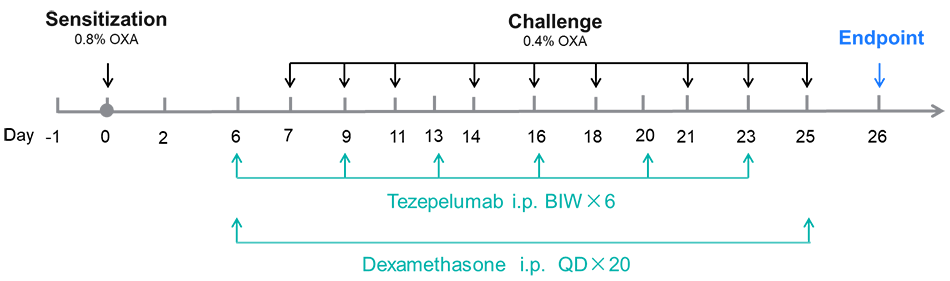
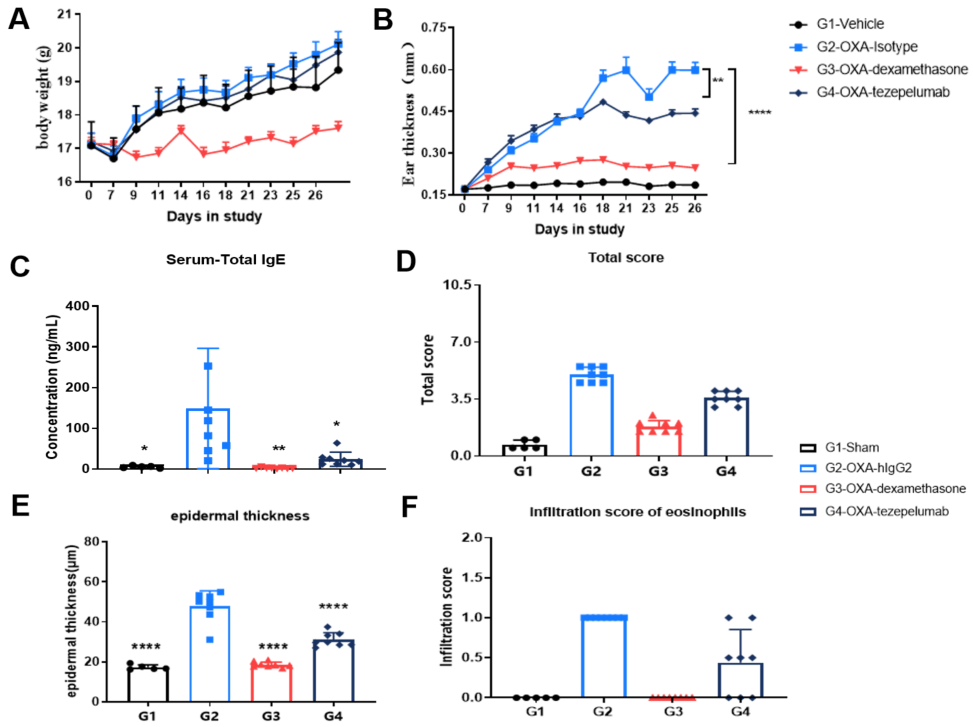
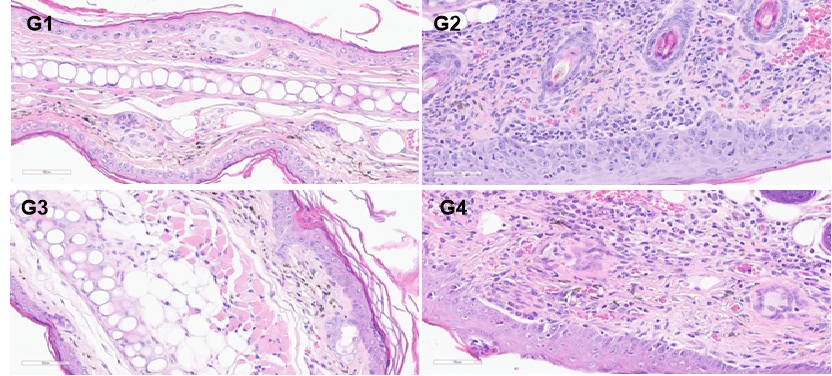
Efficacy validation on B-hTSLP/hTSLPR AD models for anti-human TSLP. A. Body weight change during treatment. B. Ear thickness change during treatment. C. Mouse total IgE in serum. D. Total score. (A-B. Two-way ANOVA; C-F. One-way ANOVA; * p< 0.05, ** p< 0.01, **** p< 0.0001).
| Readouts | ||
| Included tests | Clinical scores | Ear thickness |
| Skin histopathology (H&E) | Immune infiltration | |
| Epidermal thickness | ||
| Histology scores | ||
| Optional tests | Molecular levels | Serum total IgE |
| Protein level (Elisa or Luminex) | ||
| Behavior | Pruritus | |
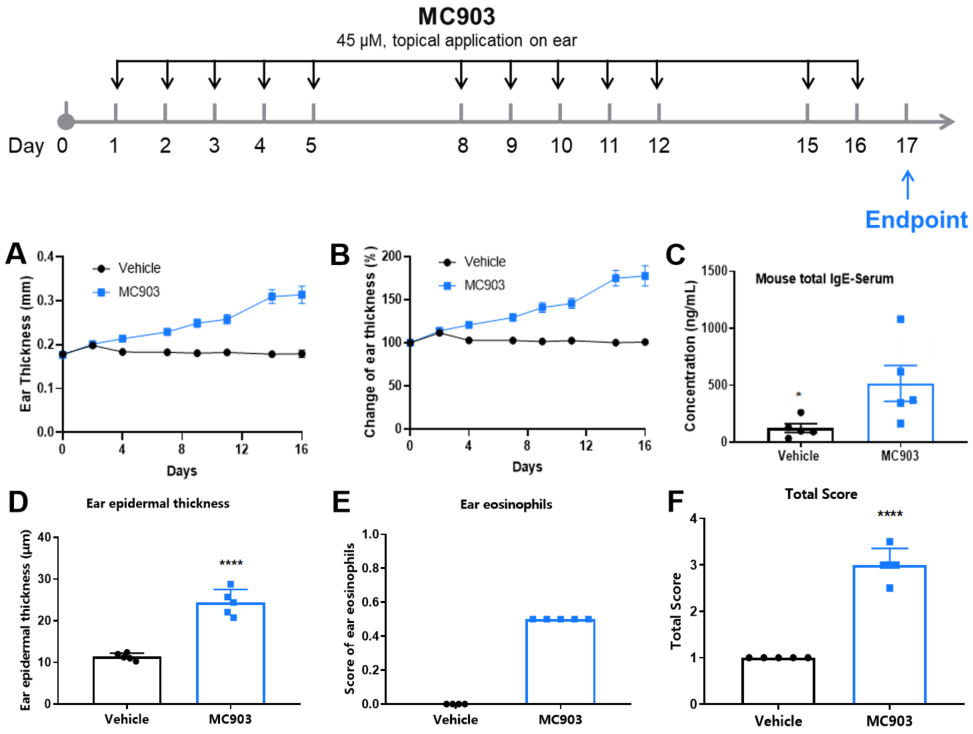
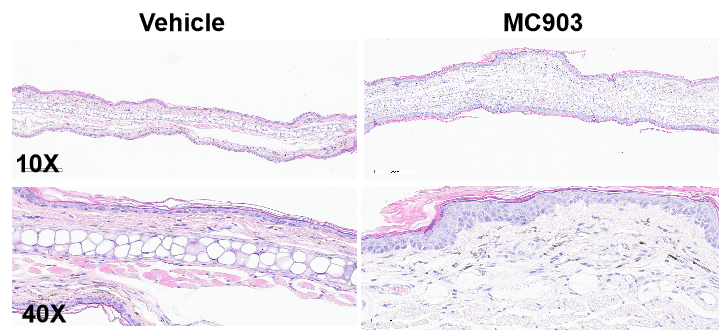
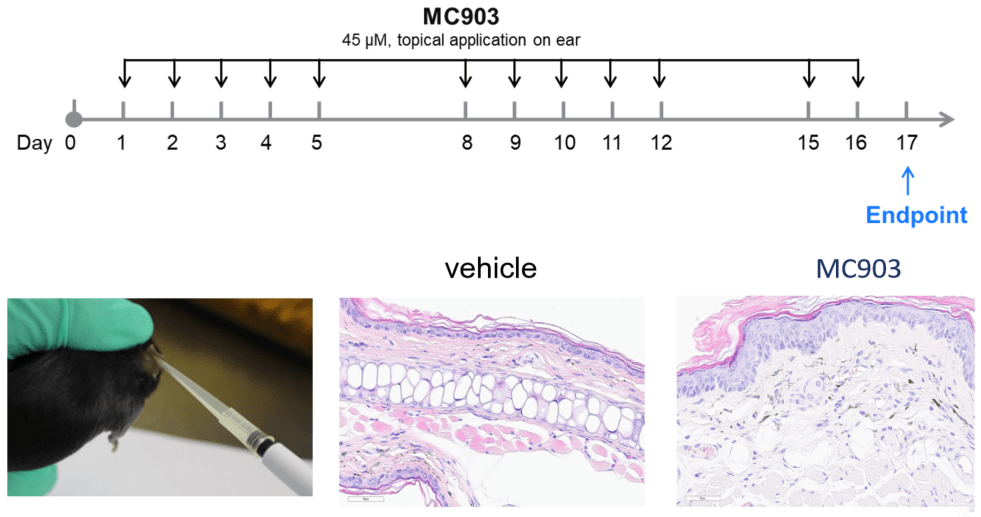
MC903-induced AD model of C57BL/6 mice. A. Ear thickness change during treatment. B. Ear thickness change (%) during treatment. C. Mouse total IgE in serum. D. Epidermal thickness score. E. Eosinophil infiltration score. F. Total score. (A-B. Two-way ANOVA; C-F. One-way ANOVA; * p< 0.05, **** p< 0.0001).
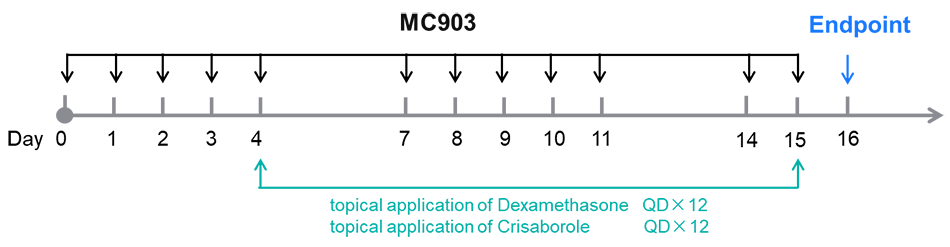
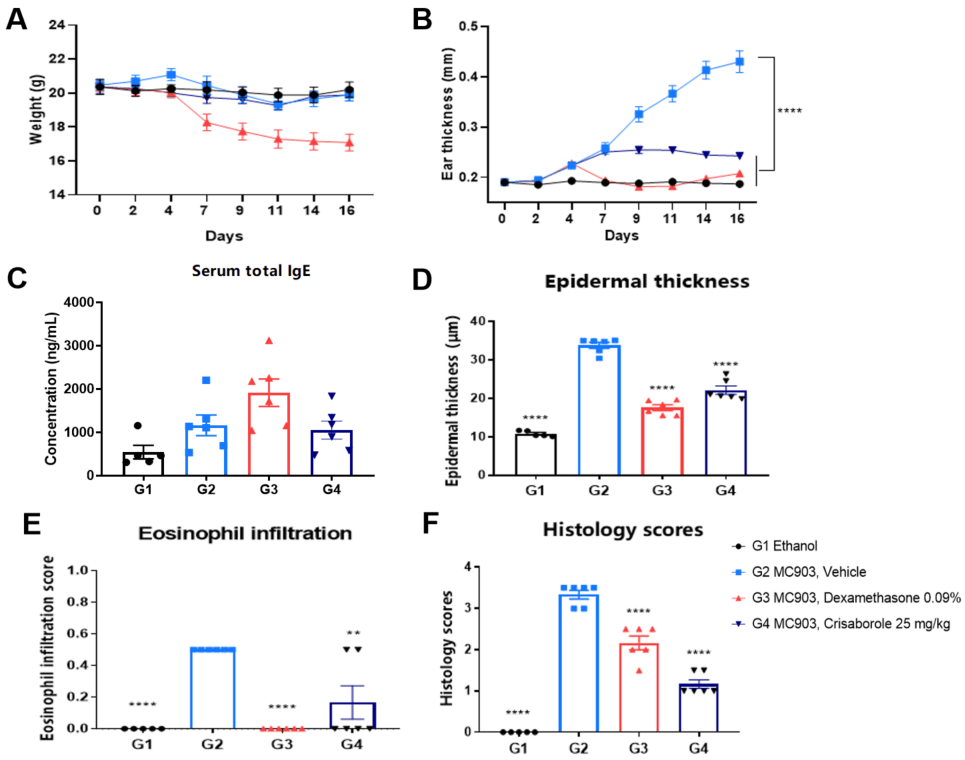
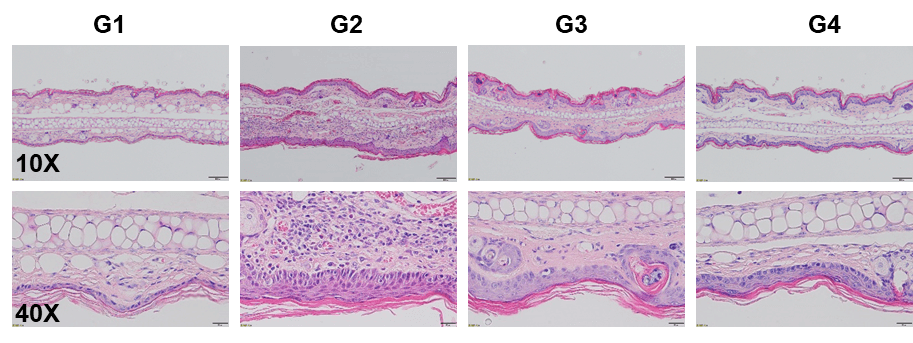
Dexamethasone and crisaborole (PDE-4 inhibitor) in MC903-induced AD model in BALB/c mice. A. Body weight change during treatment. B. Ear thickness change during treatment. C. Mouse total IgE in serum. D. Epidermal thickness score. E. Eosinophil infiltration score. F. Histology score. The data is expressed as mean ± SEM (A-F. One-way ANOVA; ** p< 0.01, **** p< 0.0001).
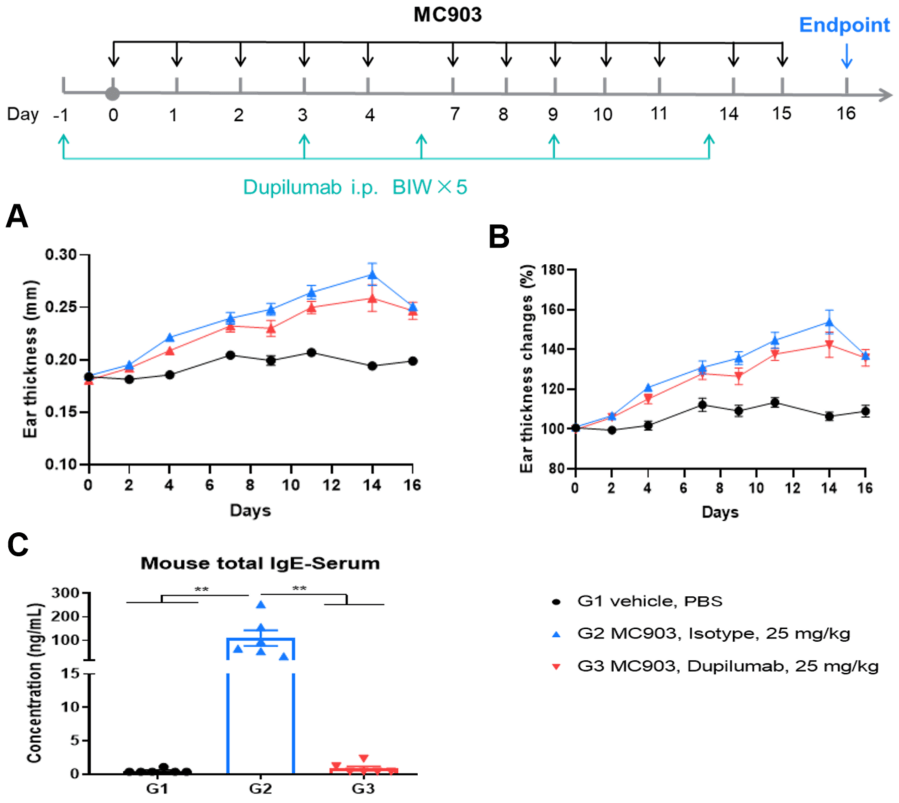
Efficacy evaluation of dupilumab(anti-IL4Rα) in MC903-induced AD model of B-hIL4/IL4Ra mice. A. Ear thickness changes during treatment. B. Ear thickness change (%) during treatment. Mouse total IgE in serum. The data is expressed as mean ± SEM. (A-B. Two-way ANOVA; C. One-way ANOVA; ** p< 0.01).