

Biocytogen has developed a series of humanized mouse models targeting single, dual, or multiple targets in response to the current market demand for innovative drug development.
on this page
Syngeneic models, where tumors are transplanted into immunocompetent mice of the same genetic background, enable the study of tumor-immune interactions and the evaluation of immunotherapies in a functional immune system.
By replacing mouse homologous proteins with human proteins, gene humanized mice overcome the issue of some antibodies being unable to cross-recognize between human and mouse. This allows for the use of human or humanized antibody drugs in efficacy evaluations, addressing the previous challenge of relying solely on surrogate antibodies for efficacy assessment. It not only improves the translational rate of drug development but also significantly reduces research and development costs. Moreover, compared to human immune reconstitution models in immunodeficient mice, it offers higher cost-effectiveness and result stability.
Biocytogen has developed a series of humanized mouse models targeting single, dual, or multiple targets in response to the current market demand for innovative drug development. Additionally, based on the possible mechanisms of drug action, we have created corresponding humanized tumor cell lines to provide more comprehensive and effective efficacy evaluation disease models.
View specific case studies in humanized CD40 mice below.
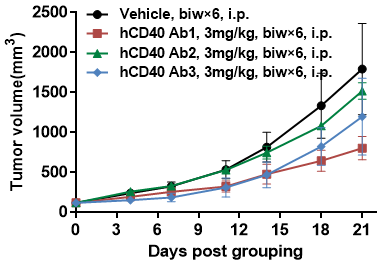
Antitumor activity of anti-human CD40 antibodies in B-hCD40 mice.
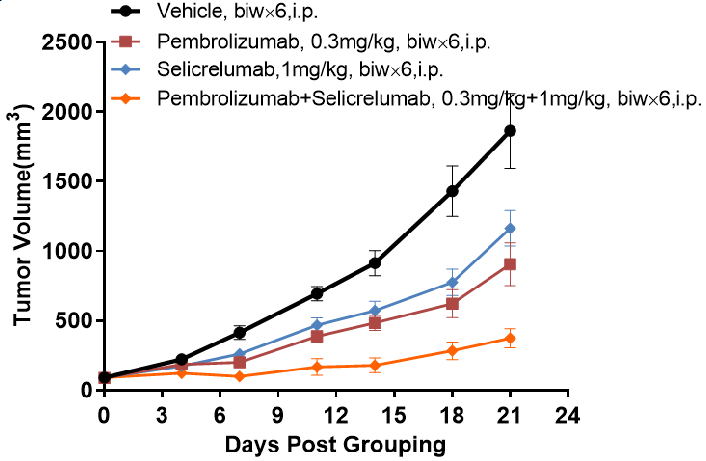
Antitumor activity of Pembrolizumab and Selicrelumab in B-hPD-1/hPD-L1/hCD40 mice.
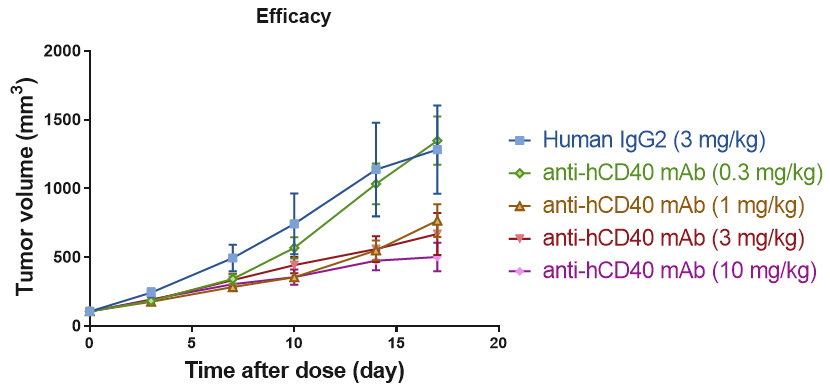
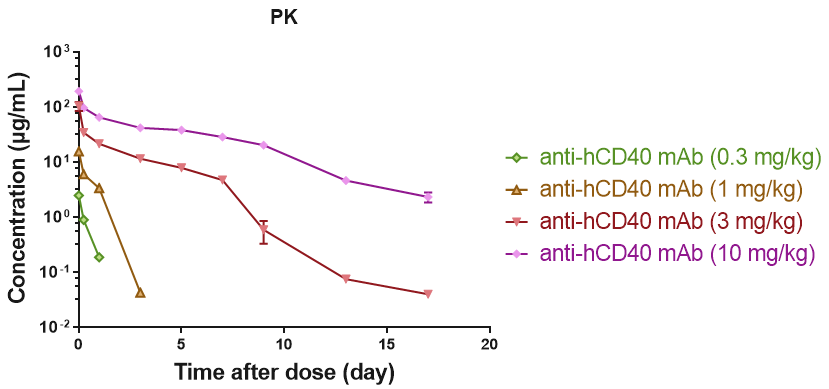
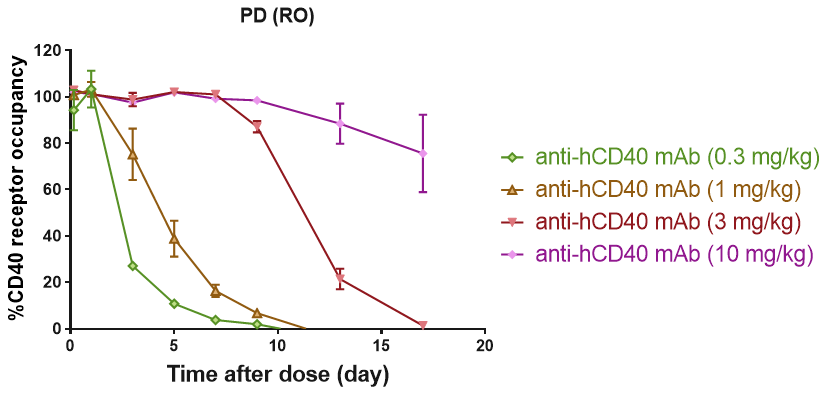
RO of B cell CD40 receptor
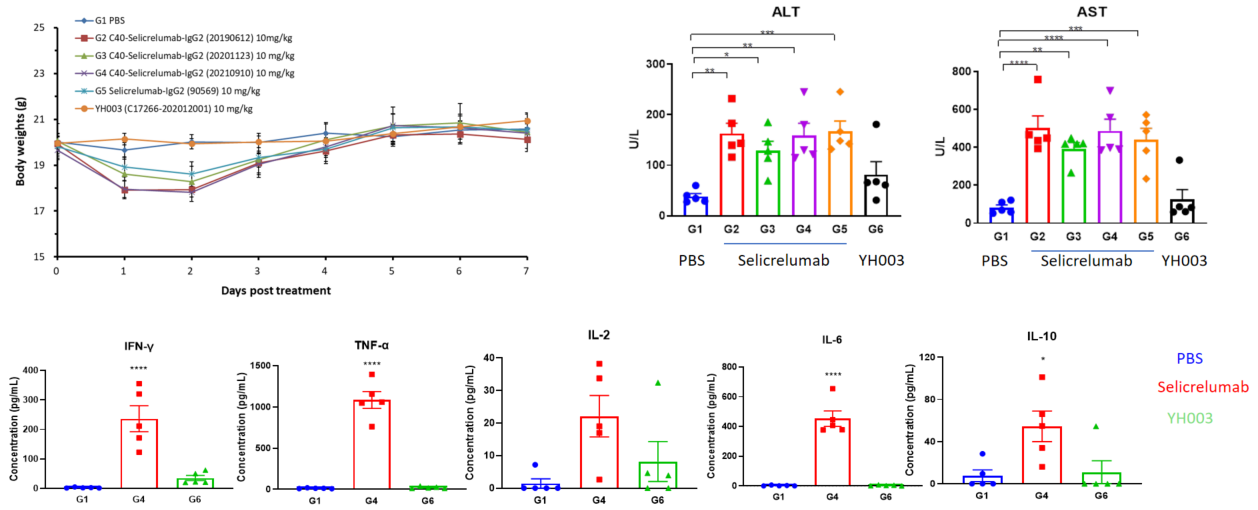
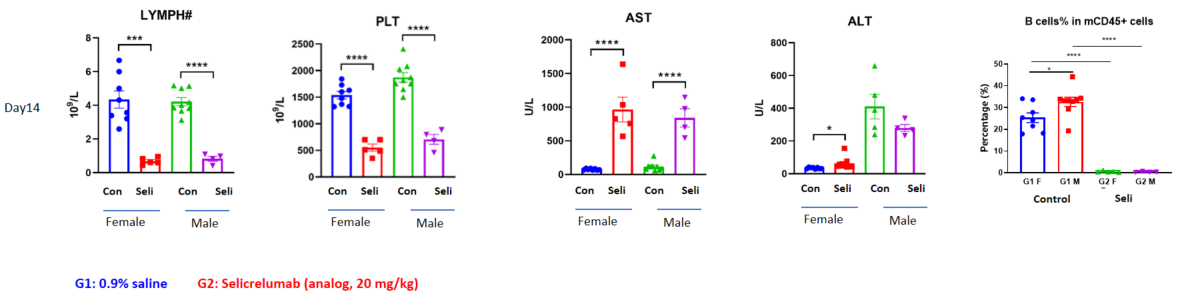
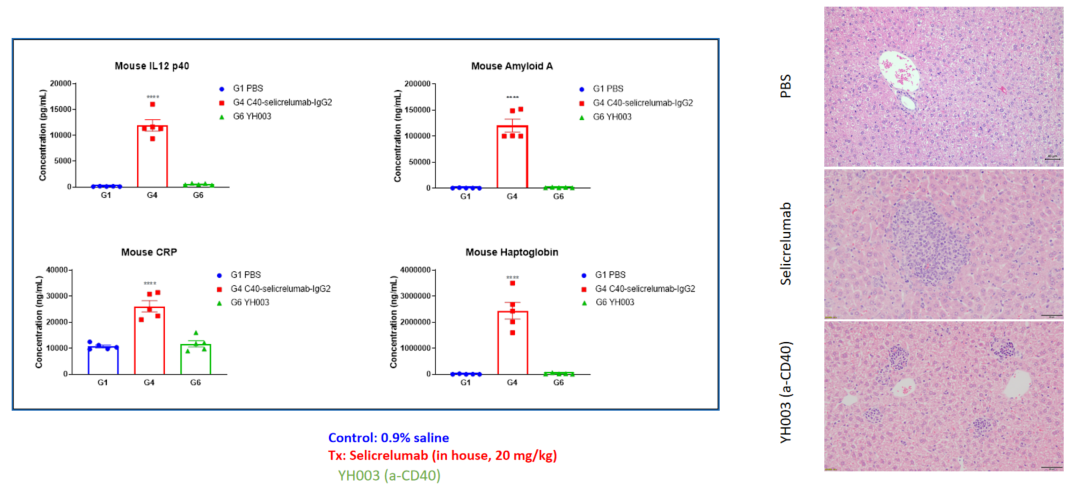
Tumor-associated antigens (TAAs) are antigenic molecules that are unique to or highly expressed on tumor cells and absent or lowly expressed on healthy cells. They are involved in the proliferation, differentiation, and migration of tumor cells, thus becoming potentially effective molecular targets of anti-tumor drugs. TAA-related drugs are able to kill tumor cells by mediating antibody-dependent cell-mediated cytotoxicity (ADCC)/complement-dependent cytotoxicitytest (CDC) and activating immune responses. The efficacy of these drugs can be evaluated via subcutaneous/in situ inoculation of TAA-humanized murine-derived cell lines in wild-type mice.
To meet the current market demand for the development of TAA-related drugs, Biocytogen has developed a set of humanized cell lines for efficacy evaluation.
Some TAA targets are expressed in healthy tissues or cells and perform important physiological functions, and drugs directing against such targets may have serious toxic side effects. Therefore, Biocytogen provides corresponding humanized animal models for drug safety evaluation.