

Biocytogen offers robust, validated asthma mouse models induced by OVA, HDM, TSLP, or Alternaria to replicate diverse asthma phenotypes. To support targeted drug development, we also provide humanized mice targeting key pathways involved in eosinophilic and allergic asthma, such as TSLP and IL-4, enabling precise evaluation of therapies against human targets.
on this page
Asthma is a chronic inflammatory disease of the airways, driven by complex genetic, epigenetic, and environmental factors. It is marked by symptoms such as shortness of breath, wheezing, coughing, and excessive mucus production—often triggered by allergens. These clinical signs stem from airway hyperresponsiveness (AHR), variable airflow obstruction, and immune-mediated inflammation. At the cellular level, airway epithelial cells, eosinophils, and various T cell subsets play central roles. In particular, Th2 cells are prominent in high eosinophilic asthma, secreting cytokines such as IL-4, IL-5, and IL-13, which drive hallmark pathologic changes.
Biocytogen offers validated murine asthma models suitable for evaluating therapeutic efficacy, such as ovalbumin (OVA), house dust mite (HDM), TSLP, and Alternaria-induced models. In the OVA-induced model, mice are sensitized via intraperitoneal ovalbumin injections and challenged with aerosolized OVA. HDM-induced models use repeated intranasal challenges over four weeks. These models consistently reproduce hallmark asthma features—including elevated IgE and eosinophil levels, airway mucus hyperproduction, and leukocyte infiltration—validated by ELISA and histological analysis.
| Readout | ||
| Included tests | Bronchoalveolar Lavage Fluid (BALF) | Cell numbers of Neutrophils, eosinophils, and macrophages |
| Serum | IgE level | |
| Histopathology | Bronchial mucus | |
| Immune infiltration | ||
| Histology scores | ||
| Optional tests | BALF | Total IgE, IL-4, IL-5, IL-13, TARC… |
| Lung tissue homogenate | IL-4, IL-5, IL-13, TARC… | |
| Lung tissue | IHC | |
| Airway function testing | Enhanced Pause (Penh) | |
Immune Cell Infiltration in Bronchoalveolar Lavage Fluid (BALF) of Asthmatic Mice
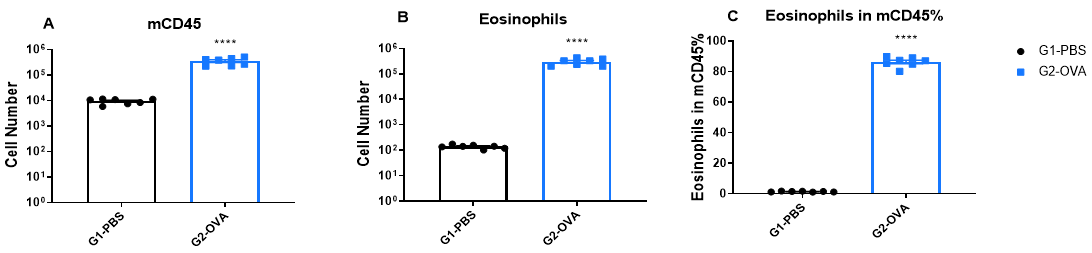
Increased Immune Cell number in BALF of OVA-induced mice compared with controls. BALF was collected at the end of the experiment and CD45+ cells number (A), eosinophils number (B) and the percent of eosinophils in CD45+ cells (C) were measured by flow cytometry.
IgE Induction in Serum of Asthmatic Mice
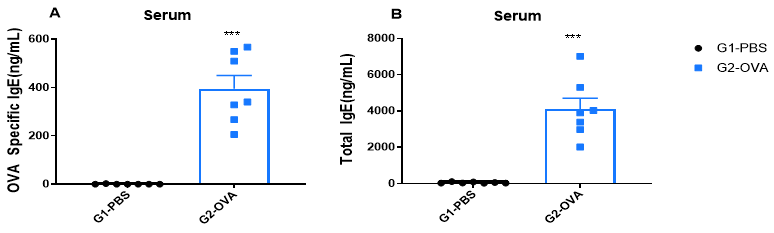
Increased IgE levels in serum of OVA-induced mice compared with controls. Serum was isolated at the end of the experiment and concentrations of OVA-specific IgE (A) and serum total IgE (B) were measured using ELISA.
Airway Histology in Asthmatic Mouse Model
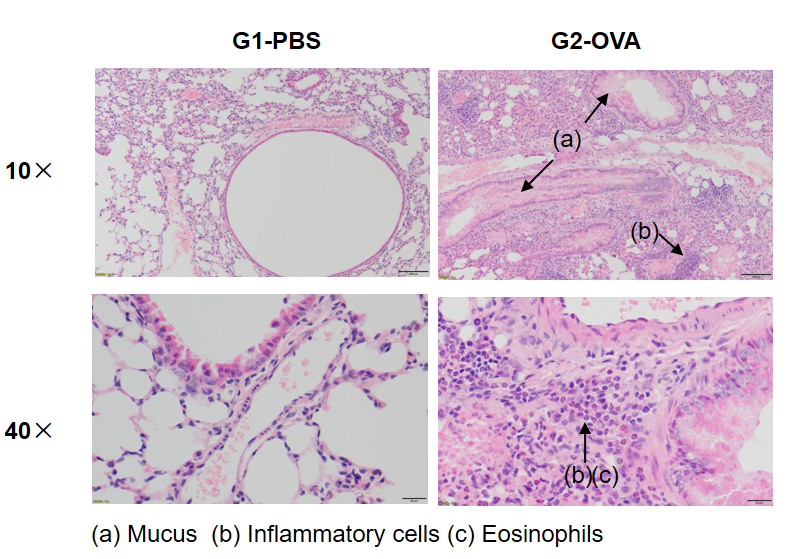
OVA successfully induces asthma-related pathology in wild-type C57BL/6 mice. H&E staining of lung tissue shows asthma-related pathology in OVA-treated (G2) mice, including vascular and peribronchial inflammation (b) and mucus (a) accumulation in some bronchi, compared to the untreated (G1) group.
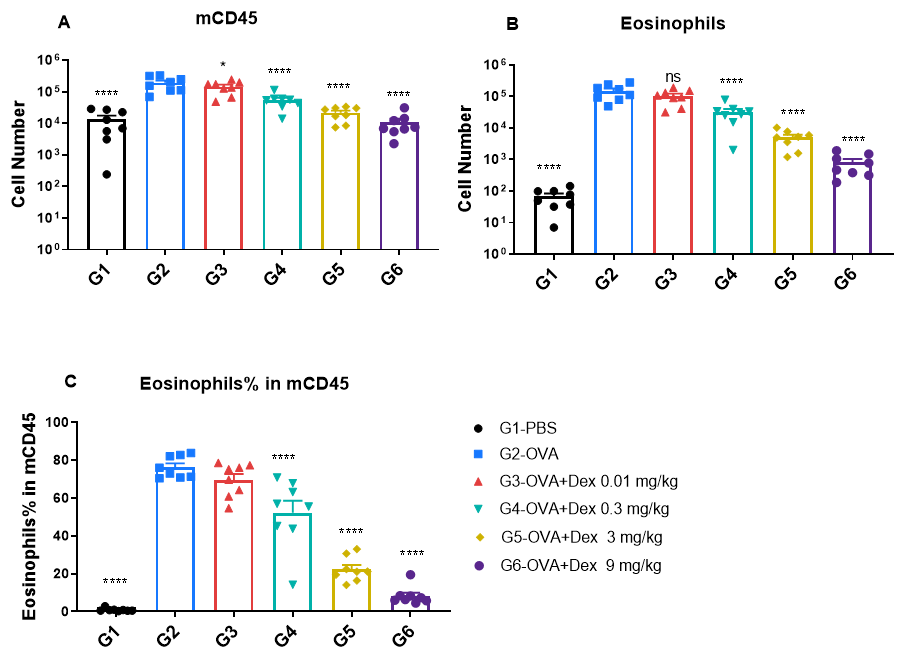
Quantification of immune cells in bronchoalveolar lavage fluid (BALF) of OVA-induced asthmatic BALB/c mice. Asthma was induced in wild-type BALB/c mice by ovalbumin (OVA) sensitization and challenge. (A) Total CD45⁺ leukocyte count in BALF; (B) eosinophil count in BALF; and (C) frequency of eosinophils among CD45⁺ cells. Mice in the OVA-induced asthma group (G2) exhibited significantly elevated total leukocytes and eosinophils compared to PBS-treated controls (G1), confirming successful model induction. Dexamethasone treatment significantly reduced CD45⁺ cell counts and eosinophil levels in asthmatic mice relative to the untreated G2 group.
Airway Histology in Asthmatic Mouse Model
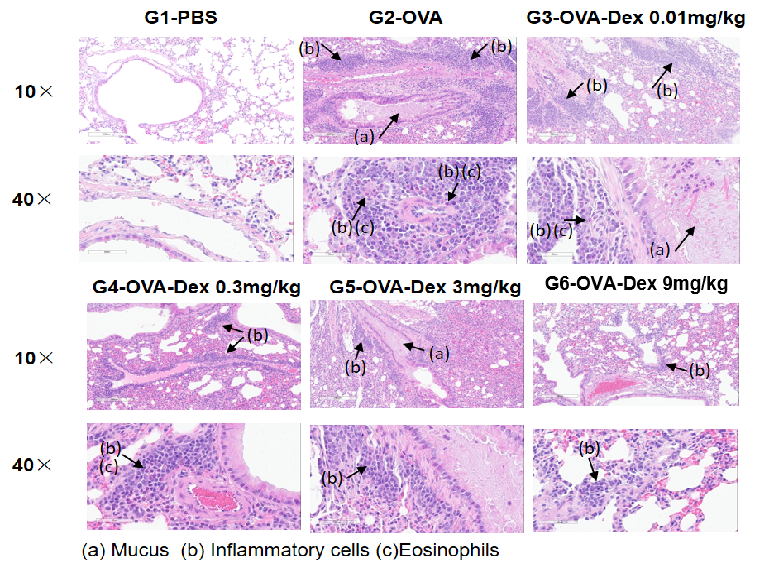
H&E staining of lung tissue in OVA-induced asthmatic mice.
Histological analysis revealed no pulmonary inflammation in the G1 control group. In the G2 (OVA-only) group, vascular and peribronchial inflammation and mucus secretion were markedly increased, confirming successful asthma model induction. Dexamethasone treatment (G3) reduced inflammatory cell infiltration and mucus production. These results validate the OVA-induced BALB/c mouse model as a platform for evaluating corticosteroid immunosuppressive efficacy.
IgE Induction in Serum of Asthmatic Mice
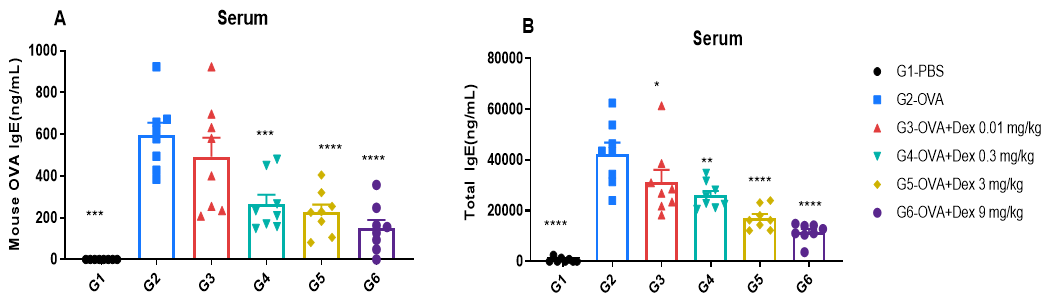
ELISA-based detection of serum IgE in OVA-induced asthmatic mice.
Serum levels of OVA-specific IgE (A) and total IgE (B) were measured by ELISA. The G2 (OVA-only) group showed significantly elevated IgE levels compared to the G1 control group, confirming successful asthma induction. Dexamethasone treatment reduced OVA-specific and total IgE levels in a dose-dependent manner, supporting its immunosuppressive efficacy.
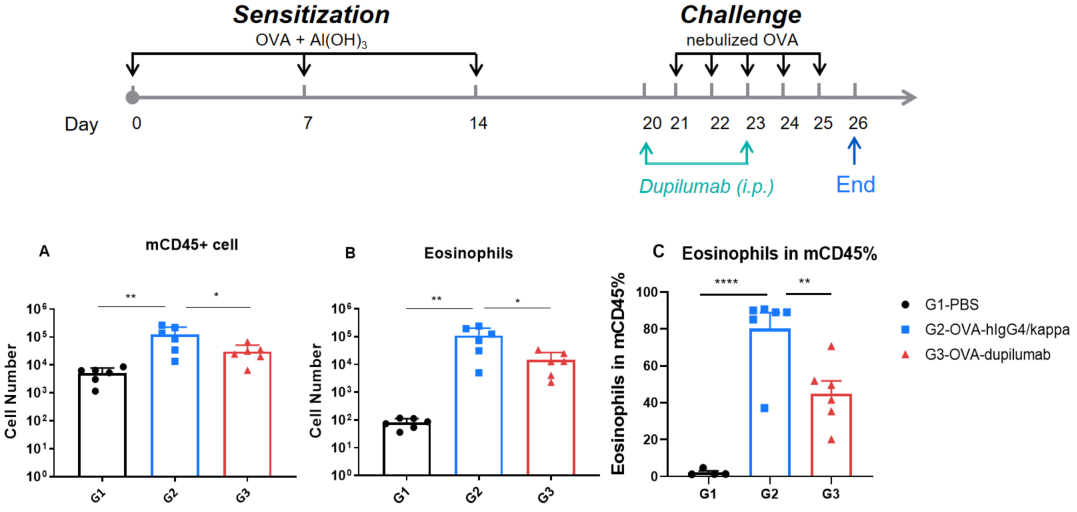
Quantification of immune cells in BALF of OVA-induced B-hIL4/hIL4RA asthmatic mice. Asthma was induced in B-hIL4/hIL4RA mice by OVA sensitization and challenge. (A) CD45⁺ cell count, (B) eosinophil count, and (C) eosinophil proportion among CD45⁺ cells in BALF. G2 (OVA-only) mice showed significantly increased leukocyte infiltration and eosinophil levels compared to G1 controls, indicating successful model induction. Treatment with in-house dupilumab markedly reduced CD45⁺ cell and eosinophil counts relative to the G2 group.

ELISA-based detection of serum IgE in OVA-induced asthmatic mouse model. Serum levels of OVA-specific IgE (A) and total IgE (B) were quantified by ELISA. G2 (OVA-only) mice exhibited significantly elevated IgE levels compared to G1 controls, confirming successful asthma model induction. Treatment with in-house dupilumab significantly reduced both OVA-specific and total IgE levels relative to the G2 group.
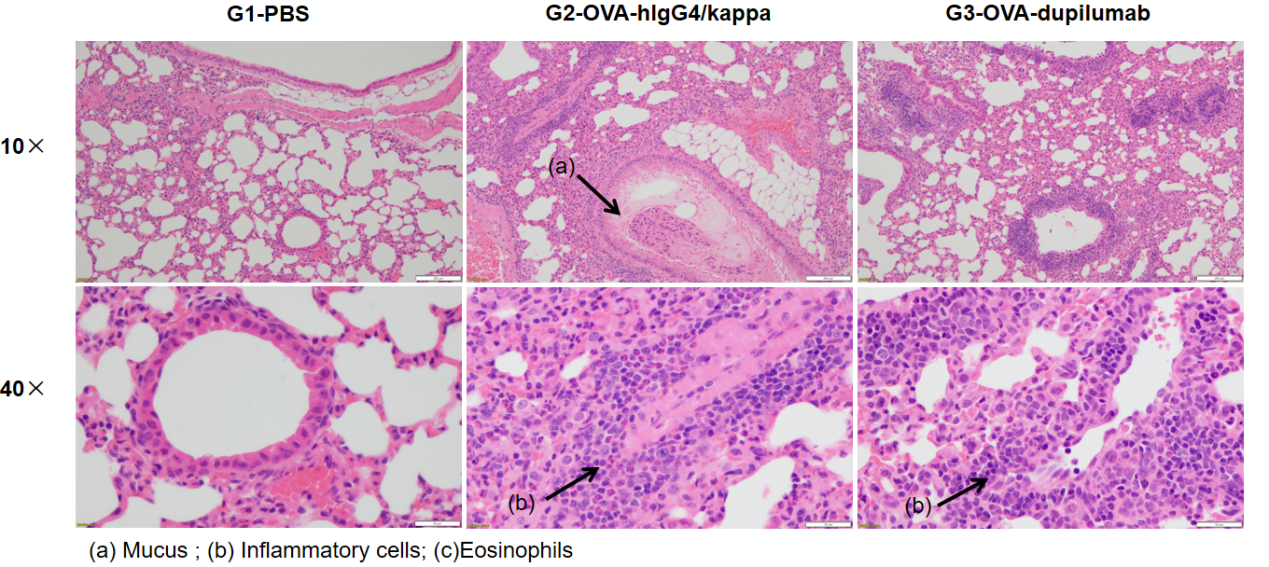
H&E staining of lung tissue in OVA-induced B-hIL4/hIL4RA asthmatic mouse model. Histopathological analysis showed no airway inflammation in G1 controls. G2 (OVA-only) mice exhibited marked vascular and peribronchial inflammation (b) and increased mucus secretion (a), confirming successful asthma model induction. Dupilumab-treated mice (G3) showed reduced inflammatory infiltration and mucus production. These findings support the use of OVA-induced B-hIL4/hIL4RA mice for evaluating anti-asthma therapeutics.

DSI Buxco FinePointe WBP: Non-Invasive Respiratory Monitoring
The FinePointe Whole Body Plethysmography (WBP) system enables precise respiratory assessment in conscious, unrestrained animals:
40-60 min for airway function testing of 2-4 mice, one test.
B-hIL4/hIL4RA mice (C57BL/6)
B-hIL4/hIL4RA mice (BaIb/c)
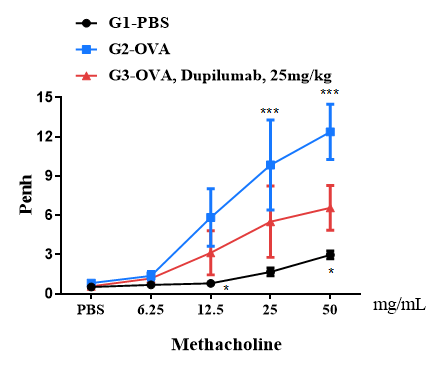
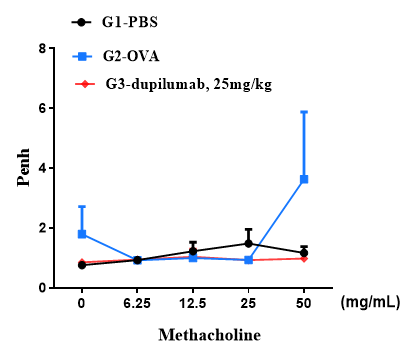
Airway responses following the exposure to increasing doses of methacholine (MCh) were measured for each mouse 24h after the final allergen or PBS exposure using the whole-body plethysmography. The y-axis represents the Penh absolute value. Increasing doses of methacholine were administered by aerosols.
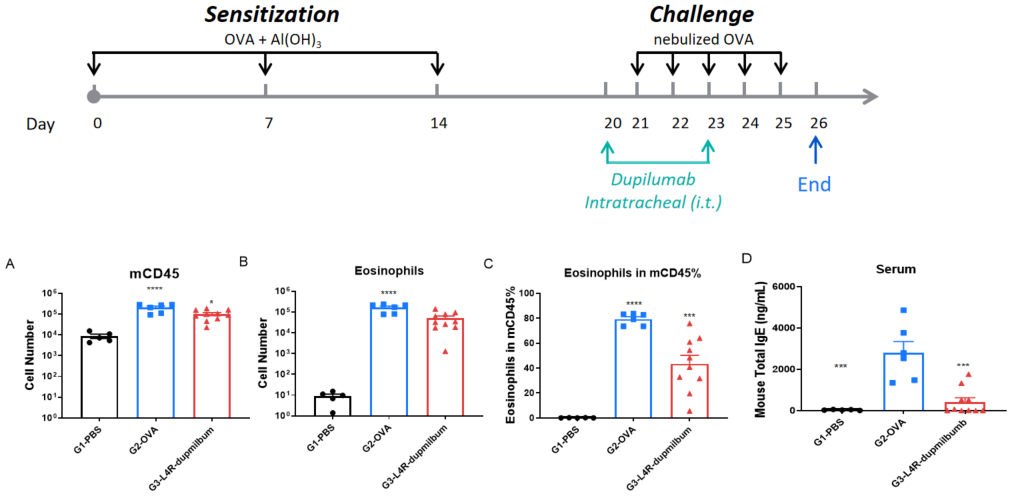
B-hIL4/hIL4RA mice were sensitized and challenged with OVA by intratracheal (i.t.) administration to induce asthma. Dupilumab was administered intratracheally. Panels show total leukocytes (A), eosinophils (B), eosinophil percentage in CD45⁺ cells (C), and serum total IgE (D). Dupilumab significantly reduced airway inflammation and IgE levels.
| Readout | ||
| Included tests | Bronchoalveolar Lavage Fluid (BALF) | Cell numbers of Neutrophils, eosinophils, and macrophages |
| Serum | IgE level | |
| Histopathology | Bronchial mucus | |
| Immune infiltration | ||
| Histology scores | ||
| Optional tests | BALF | Total IgE, IL-4, IL-5, IL-13, TARC… |
| Lung tissue homogenate | IL-4, IL-5, IL-13, TARC… | |
| Lung tissue | IHC | |
| Airway function testing | Enhanced Pause (Penh) | |
Immune Cell Infiltration in Bronchoalveolar Lavage Fluid (BALF) of Asthmatic Mice
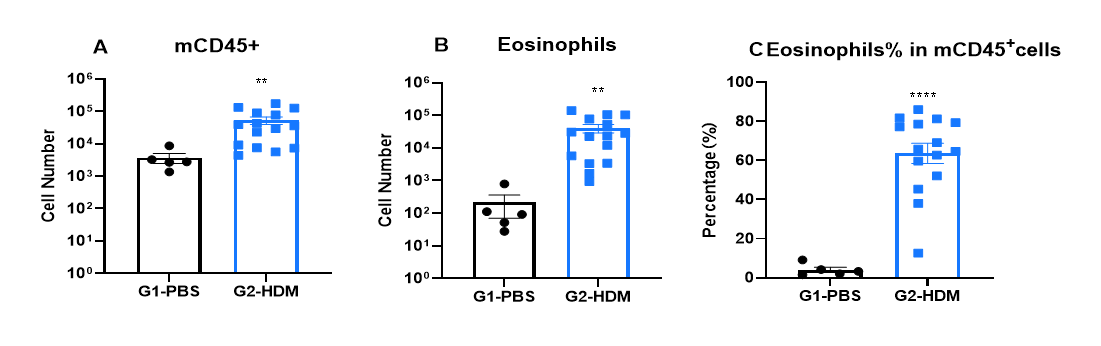
Immune cell profiling in BALF of OVA-induced IL4 and IL4 receptor humanized (B-hIL4/hIL4R) asthmatic mice. Asthma was induced in B-hIL4/hIL4RA mice via OVA sensitization and challenge. (A) CD45⁺ leukocyte count, (B) eosinophil count, and (C) eosinophil percentage among CD45⁺ cells in BALF were assessed. G2 (OVA-only) mice showed significantly increased leukocyte infiltration and eosinophilia compared to G1 controls, confirming successful model induction. In-house dupilumab treatment significantly reduced CD45⁺ cells and eosinophil levels relative to the G2 group.
IgE Induction in Serum of Asthmatic Mice
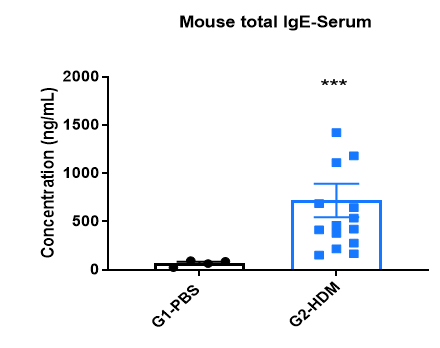
Increased IgE levels in serum of HDM-induced mice compared with controls. Serum was isolated at the end of the experiment and concentrations of total IgE were measured using ELISA.
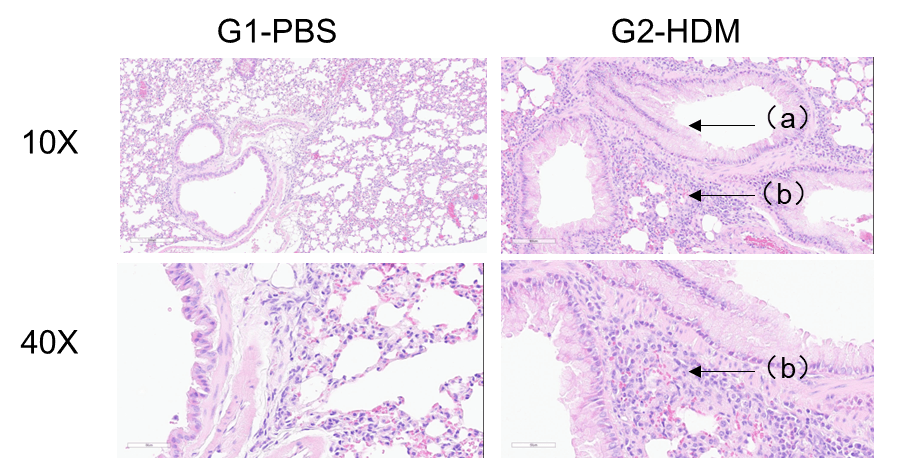
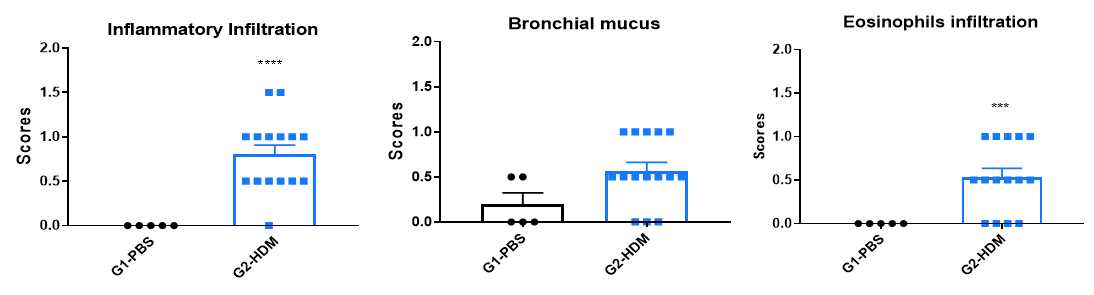
H&E staining of lung tissue in HDM-induced asthmatic C57BL/6 mice. Compared to G1 controls, G2 mice treated with house dust mite (HDM) exhibited characteristic asthma pathology, including vascular and peribronchial inflammatory cell infiltration (b) and mucus production (a). These findings confirm that HDM successfully induces asthma in wild-type C57BL/6 mice.
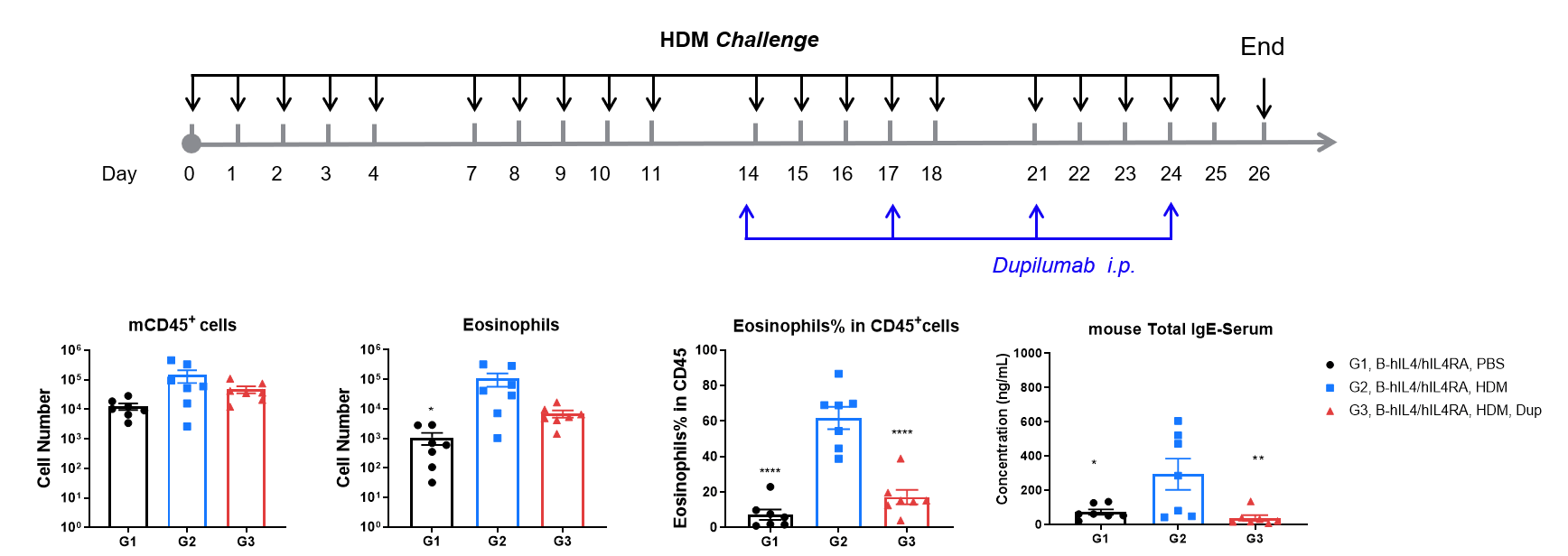
Efficacy of anti-human IL-4R antibody (dupilumab) in HDM-induced asthma model of IL-4 and IL-4 receptor humanized (B-hIL4/hIL4R) mice. Following HDM sensitization and challenge, G2 mice showed significantly increased CD45⁺ leukocyte counts, eosinophil numbers, and eosinophil proportions in bronchoalveolar lavage fluid (BALF) compared to G1 controls, indicating successful asthma model induction. Treatment with in-house dupilumab (25 mg/kg) significantly reduced CD45⁺ cells and eosinophils. At endpoint, serum total IgE levels measured by ELISA were also elevated in G2 and significantly reduced after dupilumab administration, confirming therapeutic efficacy of IL-4R blockade.
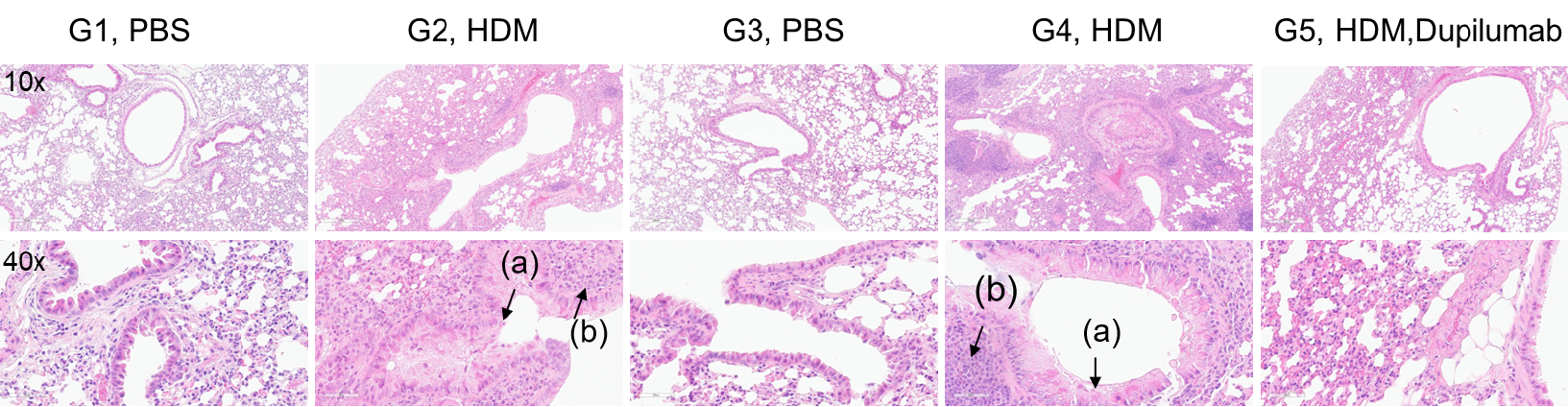
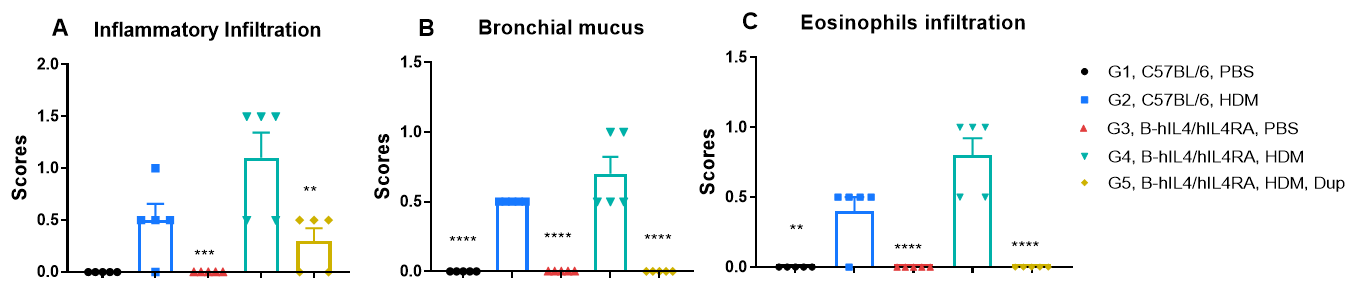
H&E staining of lung tissue in HDM-induced asthmatic mice. Compared to G1 (C57BL/6) and G3 (B-hIL4/hIL4RA) untreated controls, G2 (C57BL/6) and G4 (B-hIL4/hIL4RA) mice treated with house dust mite (HDM) exhibited hallmark asthma pathology, including peribronchial and perivascular mixed inflammatory cell infiltration (b) and mucus accumulation (a) in the bronchi. Dupilumab treatment significantly reduced these pathological features, demonstrating the therapeutic efficacy of anti-IL-4R blockade in an HDM-induced allergic asthma model.
| Readout | ||
| Included tests | Bronchoalveolar Lavage Fluid (BALF) | Cell numbers of Neutrophils, eosinophils, and macrophages |
| Serum | IgE level | |
| Histopathology | Bronchial mucus | |
| Immune cell infiltration | ||
| Histology scores | ||
| Optional tests | BALF | Total IgE, IL-4, IL-5, IL-13, TARC… |
| Lung tissue homogenate | IL-4, IL-5, IL-13, TARC… | |
| Lung tissue | IHC | |
| Airway function testing | Enhanced Pause (Penh) | |
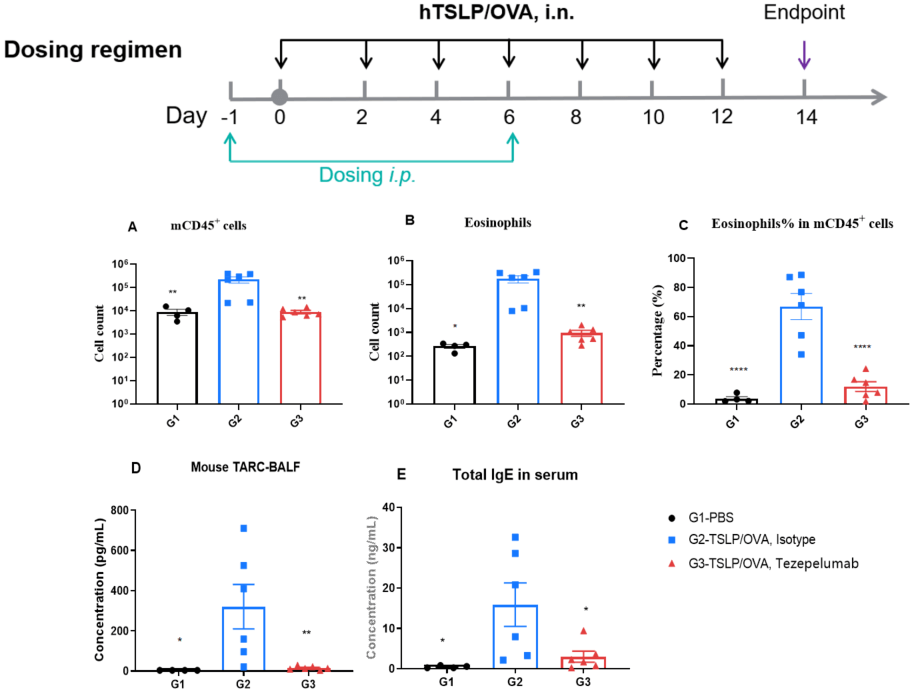
Efficacy of anti-human TSLP antibody (Tezepelumab) in hTSLP/OVA-induced asthma model of TSLP and TSLP receptor humanized (B-hTSLP/hTSLPR) mice. OVA/TSLP sensitization induced asthma in B-hTSLP/hTSLPR mice, as evidenced by significantly elevated CD45⁺ leukocyte counts, eosinophil numbers, and eosinophil proportions in bronchoalveolar lavage fluid (BALF) in G2 compared to G1 controls. Tezepelumab (in-house) treatment markedly reduced CD45⁺ cells and eosinophils. At endpoint, BALF TARC levels and serum total IgE concentrations, measured by ELISA, were also significantly increased in G2 and reduced following tezepelumab administration, confirming the therapeutic efficacy of TSLP blockade in this allergic asthma model.
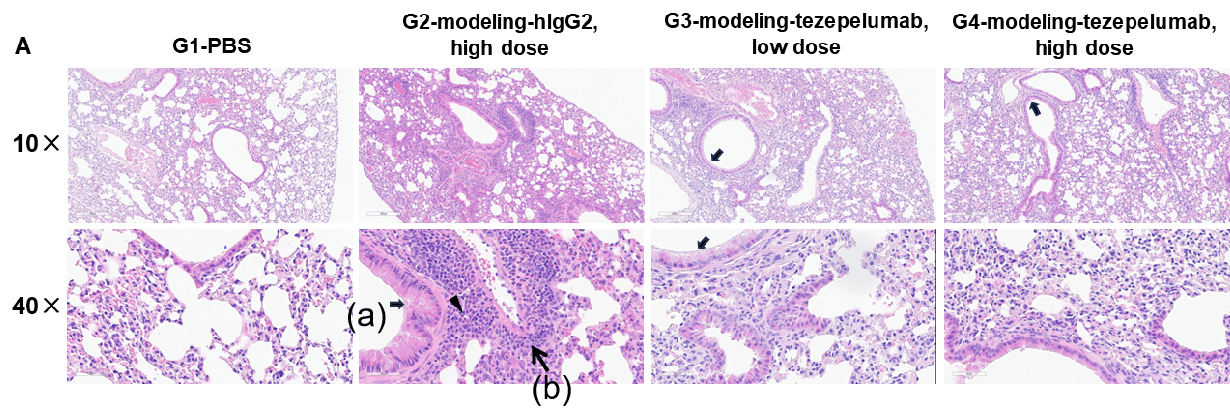
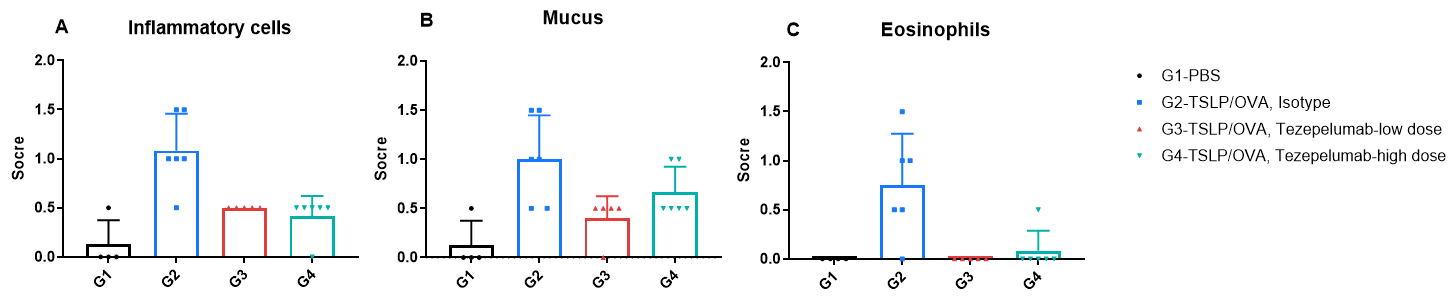
H&E staining and histopathological scoring of lung tissue in TSLP/OVA-induced asthma model of TSLP and TSLP receptor humanized (B-TSLP/TSLPR) mice. Compared to G1 (PBS-treated controls), G2 (TSLP/OVA + isotype) mice exhibited hallmark asthma pathology, including vascular and peribronchial mixed inflammatory cell infiltration (b) and mucus accumulation (a) in bronchi. Tezepelumab treatment in G3 (low dose) and G4 (high dose) reduced airway inflammation and mucus secretion in a dose-dependent manner. Increased inflammatory cell infiltration, mucus production, and eosinophil presence were observed in G2, all of which were attenuated by anti-TSLP therapy, supporting the efficacy of tezepelumab in this allergic asthma model.