

on this page
Gene therapy refers to a technique that involves the direct modification of a patient's genes to treat or prevent disease. With the rapid advancement of technologies such as vector delivery and gene editing, gene therapy has been widely applied in the research of hard-to-treat diseases like genetic disorders and cancer, showing remarkable efficacy. Methods used in gene therapy include replacing a faulty or missing gene with a functional one, inactivating a mutated gene that causes disease, or introducing a new or modified gene to help combat a disease.
Biocytogen has generated a series of gene edited mouse models tailored for gene therapy research, designed to facilitate the evaluation and accelerate the development of gene therapies for diseases such as hemophilia and spinal muscular atrophy.
Our pharmacology platform is designed to evaluate the efficacy, safety and mechanism of action of gene therapy products in support of Investigational New Drug (IND) applications. Our flow cytometry platform and cell base platform support a series of in-vitro and ex-vivo studies in a quick, reliable, and reproducible way, including the evaluation of immune cell activation, tissue distribution, immunogenicity etc.
Additionally, our full-fledged team with abundant animal model resource efficiently support the efficacy evaluation of gene therapy products in vivo. Furthermore, our pathology/toxicology platform provides comprehensive drug safety evaluation during preclinical study.
| Efficacy | MOA | Safety |
|
|
|
F8, also known as AHF, FVIII (coagulation factor VIII), is a gene located on the X chromosome encoding factor VIII, which is indispensable in both the extrinsic and intrinsic coagulation pathways. It is involved in the coagulation process as a cofactor of factor IXa, while by means of Ca2+ and membrane phospholipids, a complex is formed to activate factor X, and a series of subsequent coagulation reactions are carried out. Deletion of the F8 gene leads to severe coagulopathy, forming hemophilia A with X-linked recessive inheritance, and the F8 knockout model is undoubtedly a powerful tool for studying factor VII-related coagulopathy disorders.
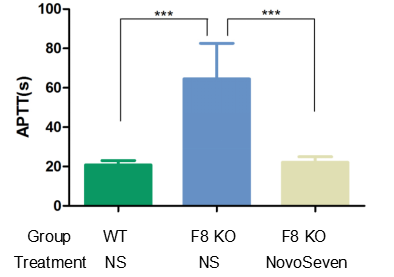
The experimental animals were randomly divided into groups and given NS(normal saline) or 1 mg/kg NovoSeven by tail vein injection, 30 minutes after injection of the drug, blood was collected from the abdominal aorta to detect the blood coagulation index: activated partial thromboplastin time APTT.
The results showed that APTT values in F8 KO mice were much higher than those in WT mice, and APTT returned to normal values after injection of NovoSeven (recombinant human coagulation factor VIIa).
The results showed that B-F8 KO mice can be used as a powerful tool for the validation of anticoagulant efficacy.
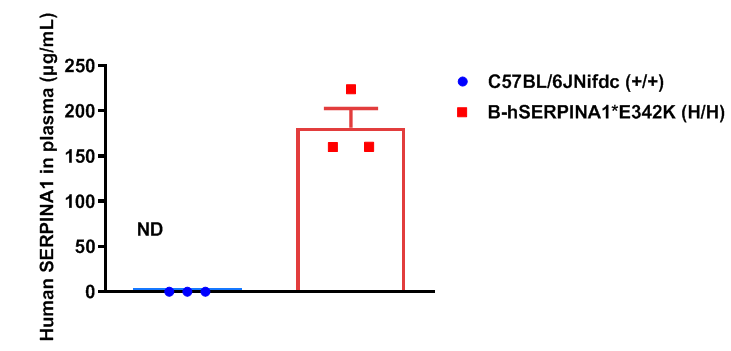
Strain specific SERPINA1 expression analysis in wild-type C57BL/6JNifdc mice and homozygous humanized B-hSERPINA1*E342K mice by ELISA. Plasma was collected from wild-type C57BL/6JNifdc mice (+/+) (male, n=3, 6-week-old) and homozygous B-hSERPINA1*E342K mice (H/H) (male, n=3, 6-week-old). Expression level of human SERPINA1 was analyzed by ELISA. Human SERPINA1 was exclusively detectable in homozygous B-hSERPINA1*E342K mice (n=3). Values are expressed as mean ± SEM.
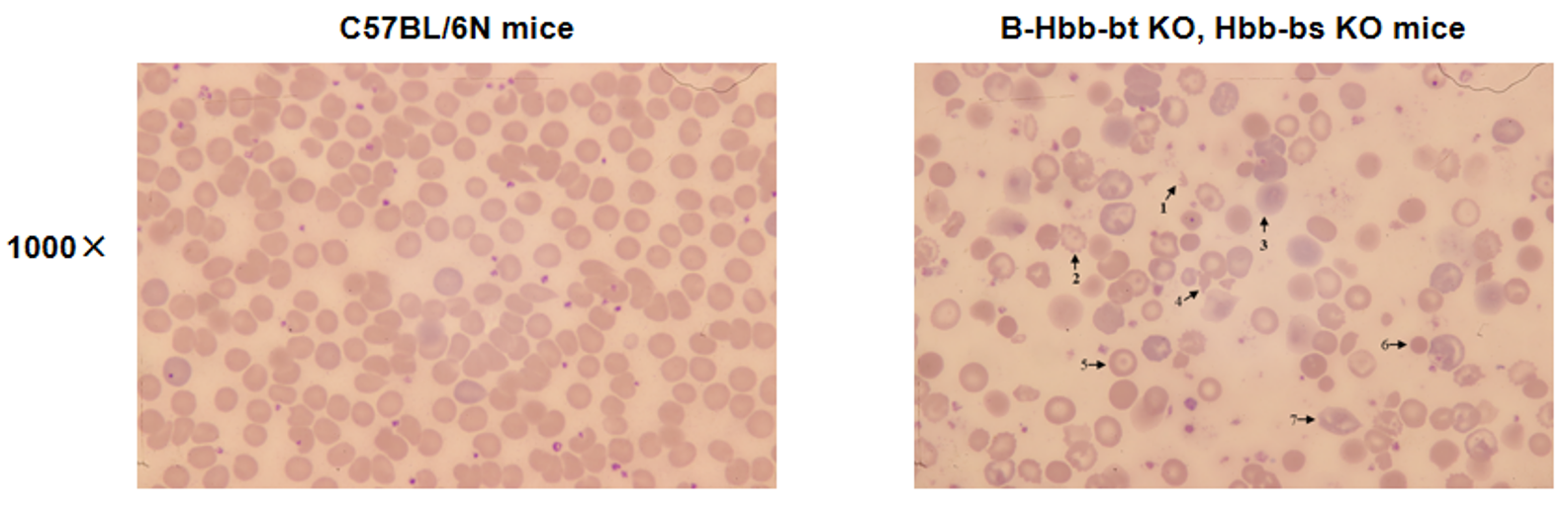
Blood smear analysis in wild-type C57BL/6N mice and heterozygous B-Hbb-bt KO, Hbb-bs KO mice. The blood smear analysis results showed that the red blood cells of heterozygous B-Hbb-bt KO, Hbb-bs KO mice (H/+) were inconsistent in size, with an enlarged central pallor. There was also a significant presence of abnormal, fragmented and nucleated red blood cells such as schistocytes (1), serrated erythrocytes (2), shadow cells or schistocytes (3), echinocytes or spiky/burr cells (4), target-like codocytes (5), spherocytes (6) and mouth-shaped stomatocytes (7).
| Assay | Method | Evaluation object | ||
| Vector | Nucleic acid | Transgene expression | ||
| Bio-distribution | qPCR,ELISA | ○ | ○ | |
| Shedding | qPCR | ○ | ○ | |
| Quantification of expression products | ELISA, MSD, WB | ○ | ○ | |
| MOA validation | Case by case | ○ | ○ | |
| Cytokine analysis | ELISA,CBA/MSD/Luminex | ○ | ○ | |
| Immune activation | FCM | ○ | ○ | |
| ADA | ELISA,MSD… | ○ | ||
| Histopathology | Pathology/Toxicology platform | ○ | ○ | |
| Immunogenicity | ELISPOT | ○ | ||
| Product Name | Product No. | Background | Action |
| B-hDMD(exon44-46, del45) mice | 114088 | C57BL/6JNifdc | |
| B-hIL13/hTSLP/hTSLPR plus mice | 113947 | C57BL/6 | |
| B-hTFR1 rats | 113101 | SD | |
| B-NDG hTHPO/hSCF mice | 113801 | B-NDG | |
| B-NDG hTROP2 mice | 113178 | B-NDG | |
| B-NDG MHC I/II DKO/hMSLN mice | 112704 | B-NDG | |
| MORE >> | |||