Type I Hypersensitivity Mouse Model
Type I hypersensitivity, or immediate hypersensitivity, is an IgE-mediated immune response triggered by allergens such as food, drugs, and environmental factors. It involves mast cell and basophil degranulation, leading to the release of inflammatory mediators like histamine and tryptase. Clinically, it manifests in diseases like atopic dermatitis (AD), allergic asthma, allergic rhinitis, allergic conjunctivitis, and most severely, systemic anaphylaxis.
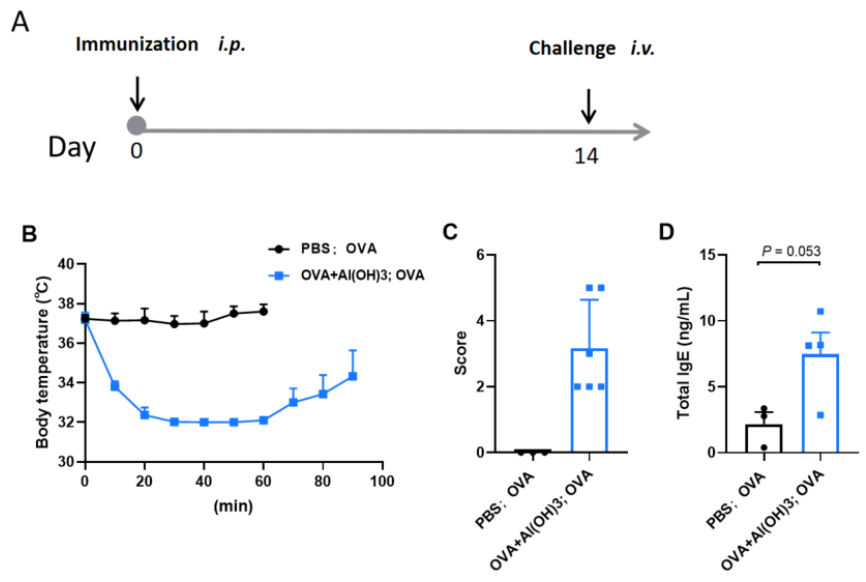
To support the development of anti-allergic therapies, Biocytogen offers a validated Active Systemic Anaphylaxis (ASA) mouse model. This model uses C57BL/6 mice sensitized with ovalbumin (OVA) and aluminum hydroxide [Al(OH)₃], followed by systemic antigen challenge, effectively replicating IgE-driven anaphylactic reactions for pharmacodynamic evaluations.
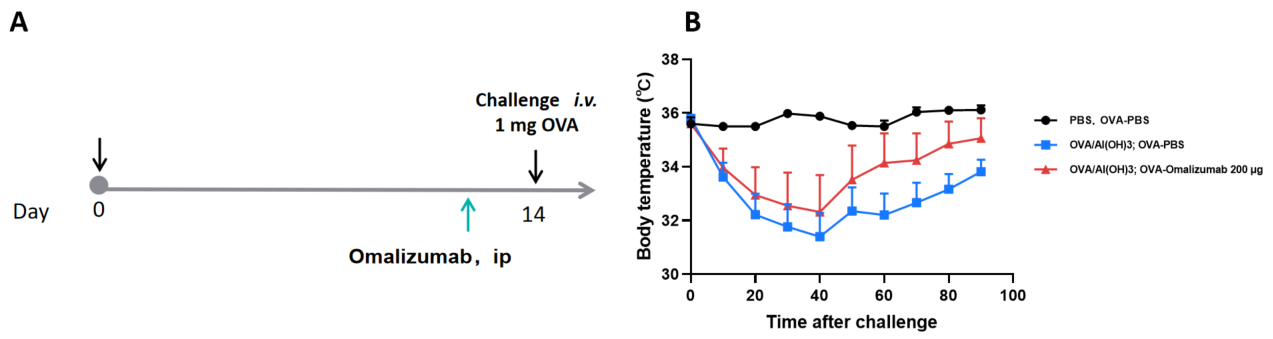
For enhanced translational relevance, Biocytogen also provides B-hIgE/hFCER1A humanized mice, which express human IgE and its high-affinity receptor (FcεRIα). This enables the study of human-like allergic responses, including the evaluation of anti-IgE therapies such as omalizumab.
Active Systemic Anaphylaxis (ASA) Mouse Model
Establishment of IBD Mouse Model
- Experimental Animals: C57BL/6 mice, 7-9 weeks old, female
- Modeling reagent: OVA/ AL(OH)3;OVA
- Modeling paradigm:
| Readout |
| Included tests |
Body temperature, |
| Score |
| Optional tests |
Histamine (ELISA) |
| Mast cell analysis |
| Mouse IgE (ELISA) |
| Anaphylactic symptom score |
| 0 |
No clinical symptoms |
| 1 |
Repetitive mouth/ear scratching and ear canal digging with hind legs |
| 2 |
Decreased activity; self isolation; puffiness around eyes and/or mouth |
| 3 |
Periods of motionless for more than 1 min; lying prone on stomach |
| 4 |
No response to whisker stimuli; reduced or no response to prodding |
| 5 |
Endpoint: tremor; convulsion; death |
OVA-induced active systemic anaphylaxis (ASA) model mimicking type I hypersensitivity. C57BL/6 mice were sensitized with OVA/Al(OH)₃ on Day 0 and challenged intravenously with OVA on Day 14. Rectal temperatures were monitored from 0–90 min post-challenge (A). OVA exposure led to decreased body temperature (B), increased anaphylaxis scores (C), and elevated serum IgE levels (D), confirming a robust IgE-mediated type I hypersensitivity response. Data shown as mean ± SEM (n = 3–6).
- Experimental Animals: B-hIgE/hFCER1A mice
- Modeling reagent: OVA/ AL(OH)3;OVA
- Modeling paradigm:
| Readout |
| Included tests |
Body temperature |
| Score |
| Optional tests |
Human IgE (ELISA) |
| Mast cell analysis |
| Histamine (ELISA) |