

Biocytogen provides models for evaluating the pharmacokinetics of antibodies, nanobodies, ADCs, small molecules, and other drugs using our state-of-the-art mouse models. Biocytogen not only offers a series of FcRn-humanized animals for assessing antibody-related PK, but has also developed albumin and FcRn dual-humanized mice to evaluate the PK of certain albumin-related nanobodies.
on this page
Preclinical pharmacokinetic (PK) studies help to understand drug behavior in the body—covering absorption, distribution, metabolism, and excretion. This information is vital for designing effective administration routes and dosage regimens. Animal models (e.g., mice, rabbits, dogs, and non-human primates) simulate human physiological and biochemical traits, aiding in the study of drug behavior and providing initial predictions about drug effects in humans.
Biocytogen provides models for evaluating the pharmacokinetics of antibodies, nanobodies, ADCs, small molecules, and other drugs using our state-of-the-art mouse models. Biocytogen not only offers a series of FcRn-humanized animals for assessing antibody-related PK, but has also developed albumin and FcRn dual-humanized mice to evaluate the PK of certain albumin-related nanobodies. Additionally, we have developed various knockout (KO) and humanized mice to assess the PK of ADCs and small molecules. Biocytogen has extensive experience in preclinical pharmacokinetics (PK) testing and bioanalysis, offering specialized pharmacokinetic analysis services based on antibody drugs.
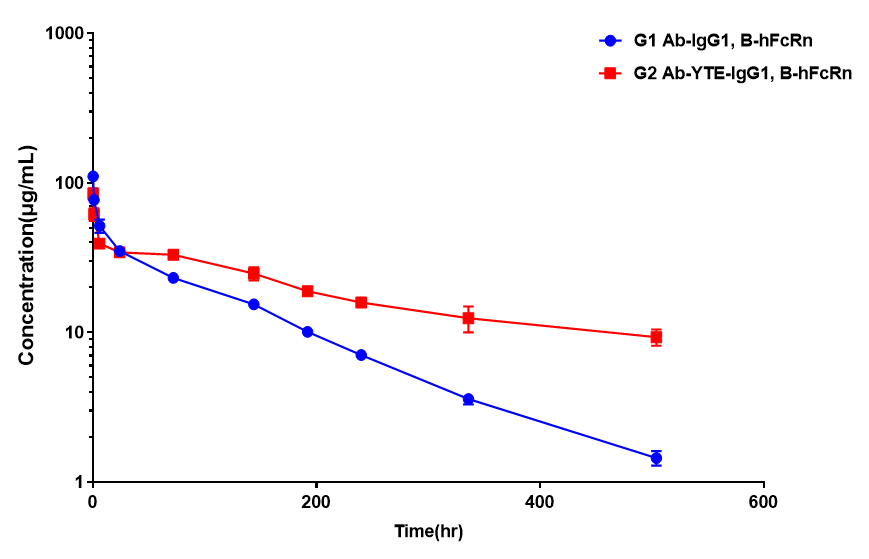
| Antibody | T1/2 (hr) |
| Ab-IgG1 | 112.87±9.78 |
| Ab-YTE-IgG1 | 261.35±27.34 |
PK study of Ab-IgG1 and Ab-YTE-IgG1 in homozygous B-hFcRn mice.
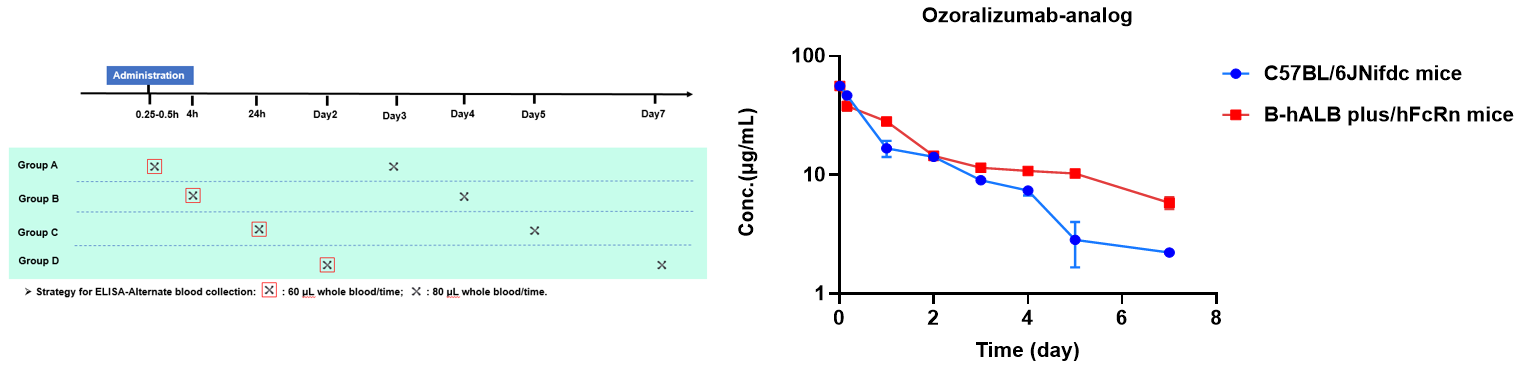
Pharmacokinetic characteristic of homozygous B-hALB plus/hFcRn mice.