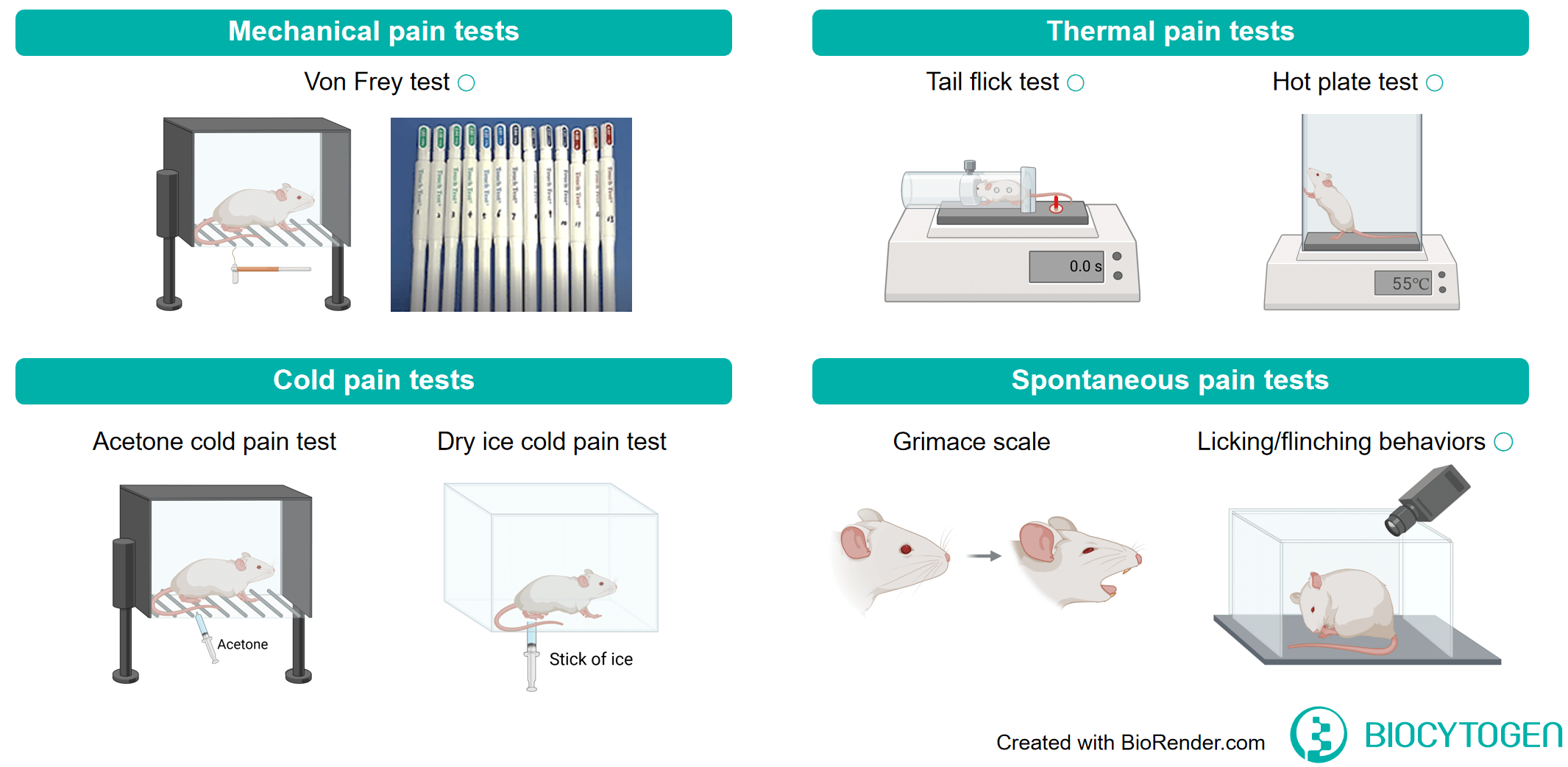
Pain and Migraine Mouse Model Introduction
Pain is an unpleasant sensory and emotional experience associated with, or resembling that associated with, actual or potential tissue damage. And pain is also a protective defense response of the mechanism to noxious stimuli. Peripheral sensory receptors detect a wide range of thermal, mechanical, and chemical stimuli from both external and internal sources. When these stimuli are intense, they can trigger acute pain. In the case of sustained injury, both peripheral and central components of the nociceptive pathway undergo significant plastic changes, amplifying pain signals and resulting in hypersensitivity. While such plasticity may be beneficial in promoting protective reflexes, persistent alterations can lead to maladaptive responses and the development of chronic pain conditions.
Pain is a common symptom that significantly affects patients' quality of life and overall health. Consequently, the development of effective analgesic drugs remains a major objective in pharmaceutical research. Mouse models of pain serve as essential tools for investigating pain mechanisms, evaluating the efficacy of therapeutic agents, and exploring strategies for pain management.
Summary of pain models in Biocytogen
| Model type |
Model names |
Modeling reagent |
Core Index |
Optional tests |
Model tested |
| Acute pain |
Tail-flick test |
- |
Tail flick latency |
- |
C57BL/6J |
| Hot-plate test |
- |
Reaction latency |
- |
C57BL/6J |
| Postoperative pain |
- |
Mechanical threshold |
Thermal sensitivity |
C57BL/6J |
| Inflammatory pain |
Formalin pain |
Formalin solution |
Time spent licking paw (mice)
Number of flinches (rat) |
- |
C57BL/6J |
| Acetic acid induced visceral pain |
Acetic acid |
Number of writhing episodes |
- |
Under development |
| CFA induced inflammatory pain |
Complete Freund's Adjuvant |
Mechanical threshold |
Thermal sensitivity
Cold sensitivity |
C57BL/6J; ICR |
| Neuropathic pain |
Spared nerve injury |
- |
Mechanical threshold |
Thermal sensitivity
Cold sensitivity |
Under development |
| Chronic constriction injury |
- |
Mechanical threshold |
Thermal sensitivity
Cold sensitivity |
Under development |
| Spinal nerve ligation |
- |
Mechanical threshold |
Thermal sensitivity
Cold sensitivity |
Under development |
| Migraine |
NTG-induced migraine |
Nitroglycerin |
Mechanical threshold |
Grimace scale |
C57BL/6J |
Summary of pain testing paradigms in Biocytogen
Results
Acute pain model: Hot plate test
- Introduction: Hot plate test is a classic nociceptive assay used to evaluate thermal pain sensitivity and the efficacy of analgesic compounds in rodents. The test records the animal's response latency to a heated surface, such as paw licking, lifting, or jumping, providing a reliable measure of pain threshold.
- Species: Rat or mouse
- Readout: Response latency
- Throughput: 60 animals per day
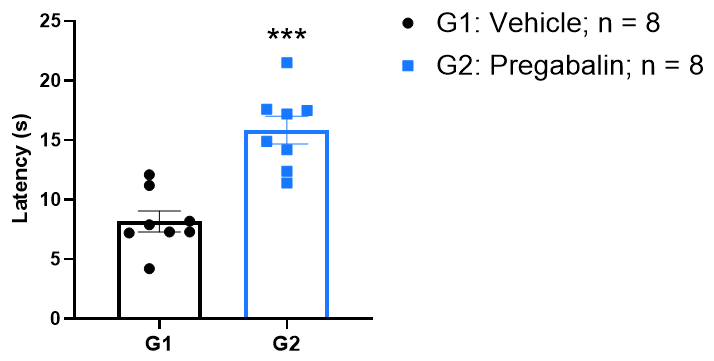
The antinociceptive effect of pregabalin in hot plate test.
Latency to response was significantly increased following pregabalin administration. ***p < 0.001, unpaired t test.
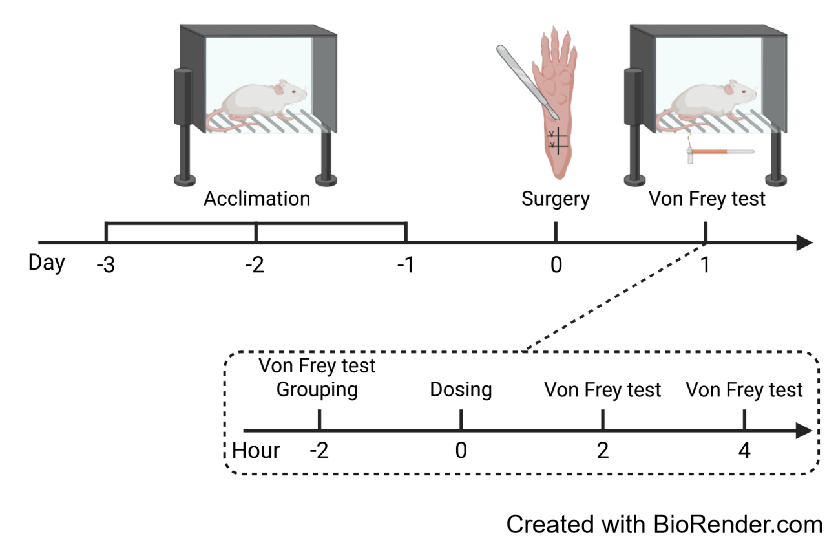
Efficacy evaluation of VX-548 and celecoxib in POP mouse model
Plantar incision surgery induced significant mechanical hyperalgesia in C57BL/6 mice. Administration of VX-548 or celecoxib significantly attenuated pain responses 2 hours post-treatment.
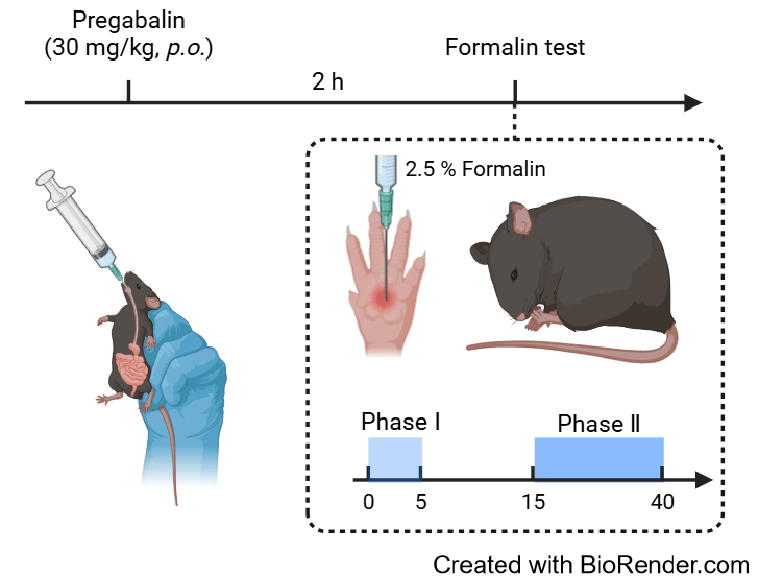
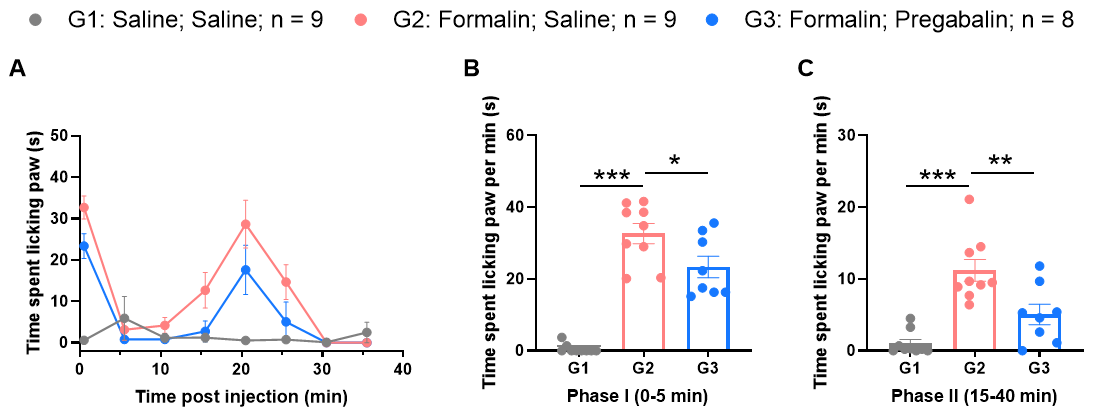
Efficacy evaluation of pregabalin in formalin test
Inhibition of biphasic pain behaviors induced by 2.5% formalin. A, Intraplantar injection of a 2.5% formalin solution caused a significant biphasic nociceptive response. B, A slight reduction in Phase I pain behaviors was observed following pregabalin administration. C, A significant reduction in Phase II nociceptive responses was observed following pregabalin administration.
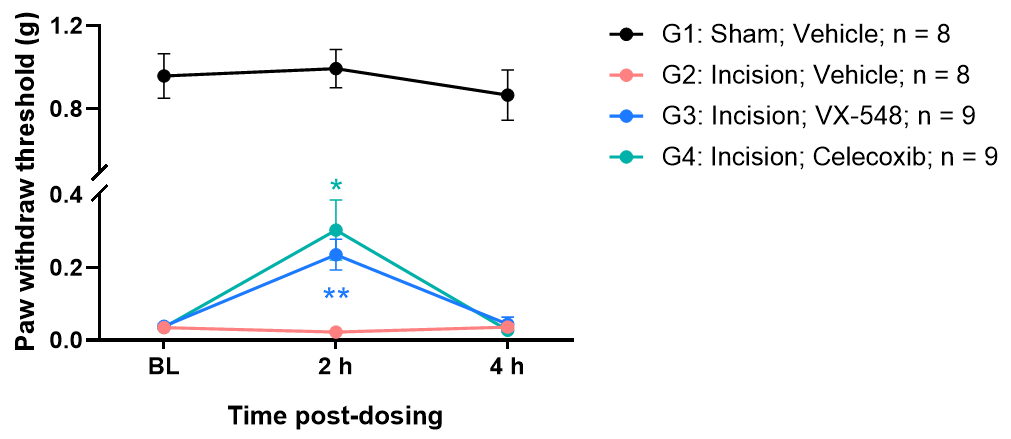
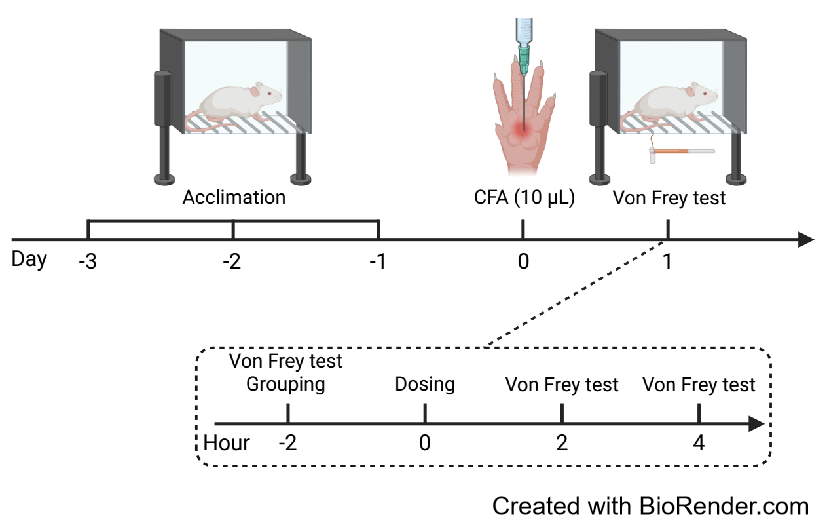
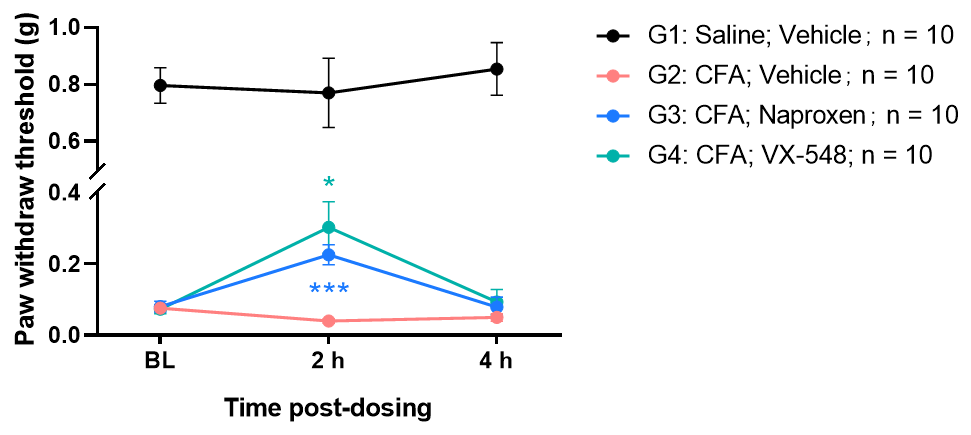
Efficacy evaluation of VX-548 and Naproxen in CFA induced pain model
Intraplantar injection of CFA induced significant mechanical hyperalgesia in mice. Administration of Naproxen or VX-548 significantly attenuated pain responses 2 hours post-treatment.
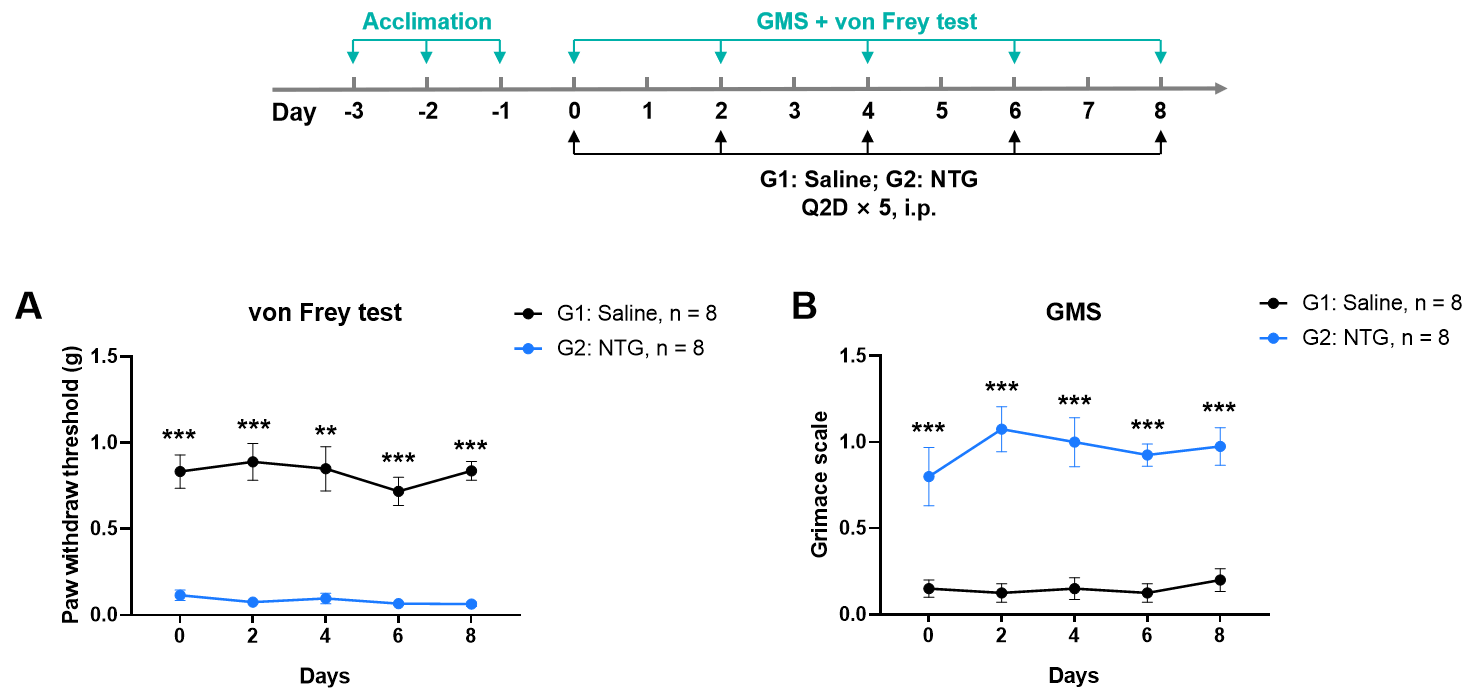
NTG induced migraine model
Nitroglycerin (NTG) induced migraine-like behaviors in mice. A, NTG injection significantly reduced paw withdraw threshold, indicating mechanical hypersensitivity. B, NTG treatment increased facial grimace scores, suggesting the presence of spontaneous pain.