

Biocytogen provides comprehensive pharmacodynamic (PD) and pharmacokinetic (PK) services to support drug discovery and development. With access to a wide range of advanced technology platforms, we deliver precise and reliable data to meet your research needs.
on this page
Mechanistic and functional data on drug efficacy and safety are essential for successful IND filings. Our scientists offer customized, professional study designs tailored to meet the specific needs of your drug development process.
Pharmacodynamic (PD) services include:
Pharmacokinetic (PK) services include:







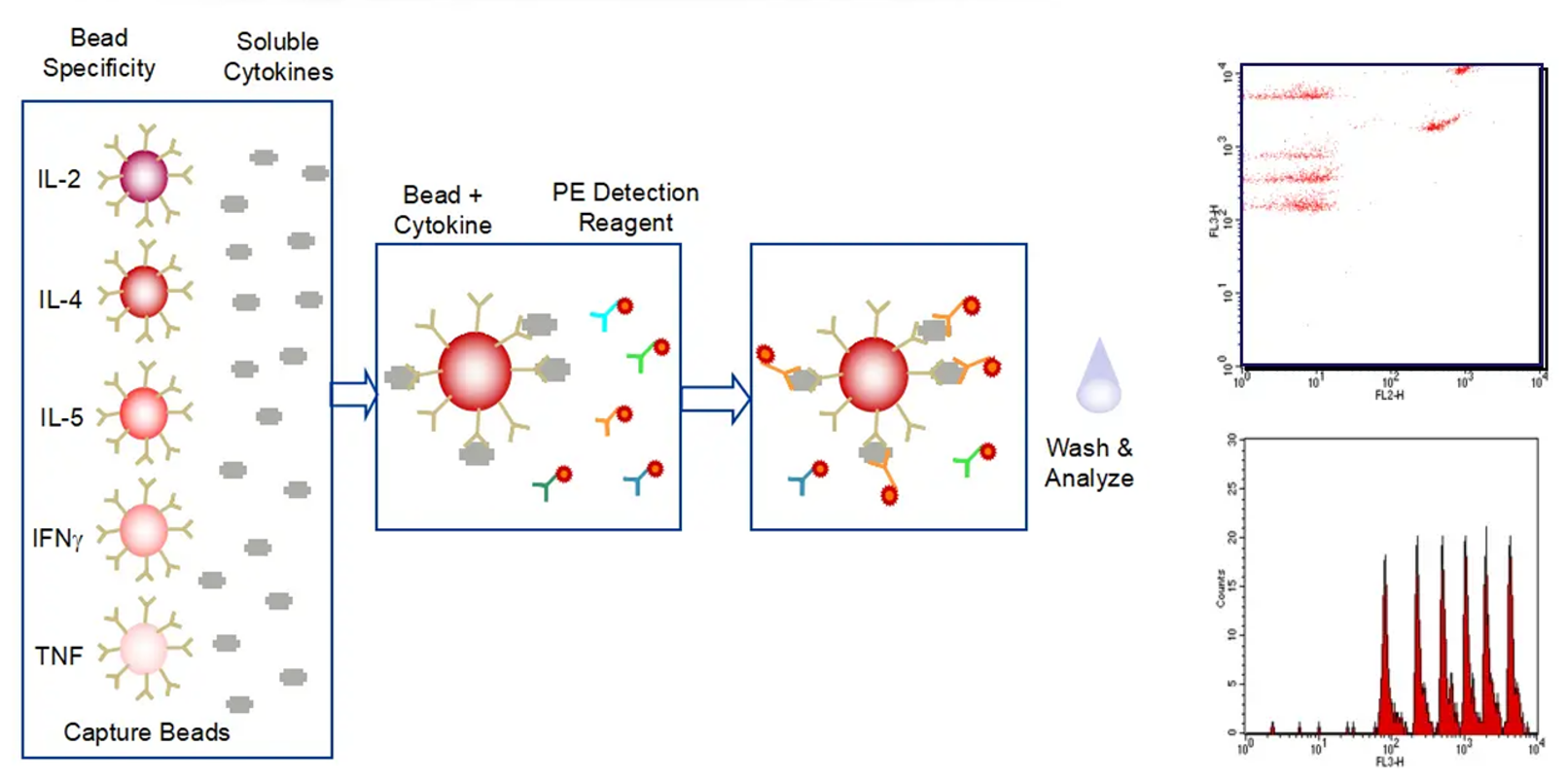
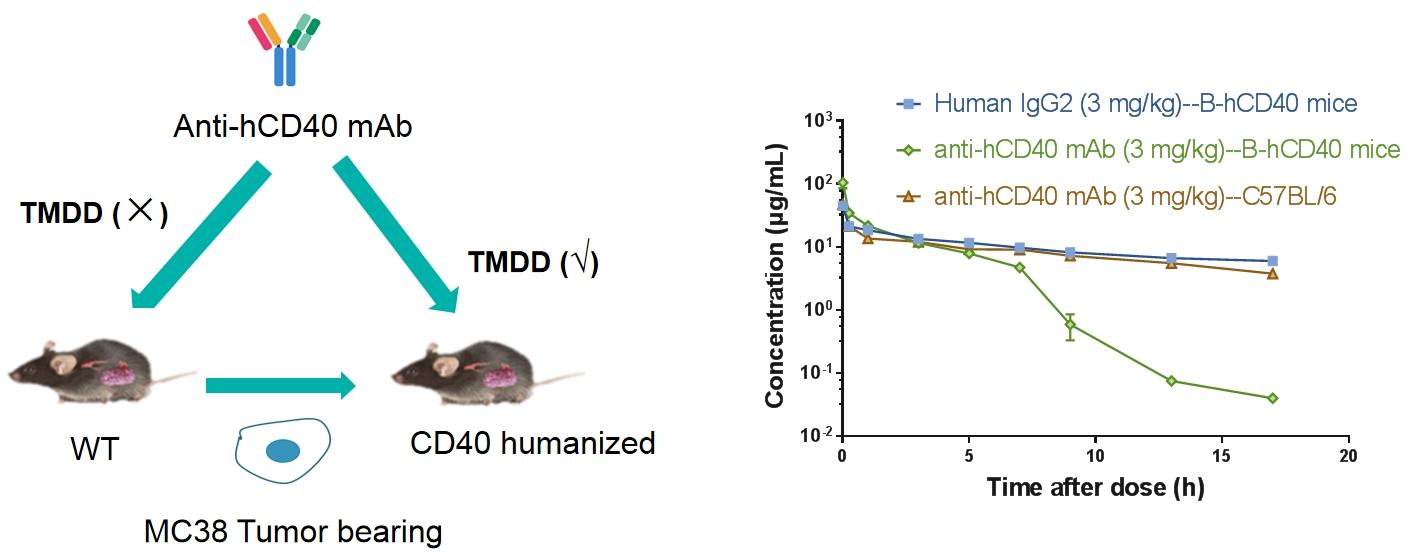
Receptor occupancy (RO) is a key pharmacodynamic indicator in the development of antibody drugs. RO analysis evaluates the binding of a test article to its target on specific cells at various time points under a given dose. By integrating RO data with pharmacokinetic and efficacy data, researchers can better understand the dose-response relationship. Flow cytometry is our primary method for conducting receptor occupancy studies, using competitive antibodies, non-competitive antibodies, and anti-drug fluorescent secondary antibodies. By examining different detection conditions, we measure signals for free receptors, bound receptors, and total receptors, allowing for precise calculation of the receptor occupancy rate.
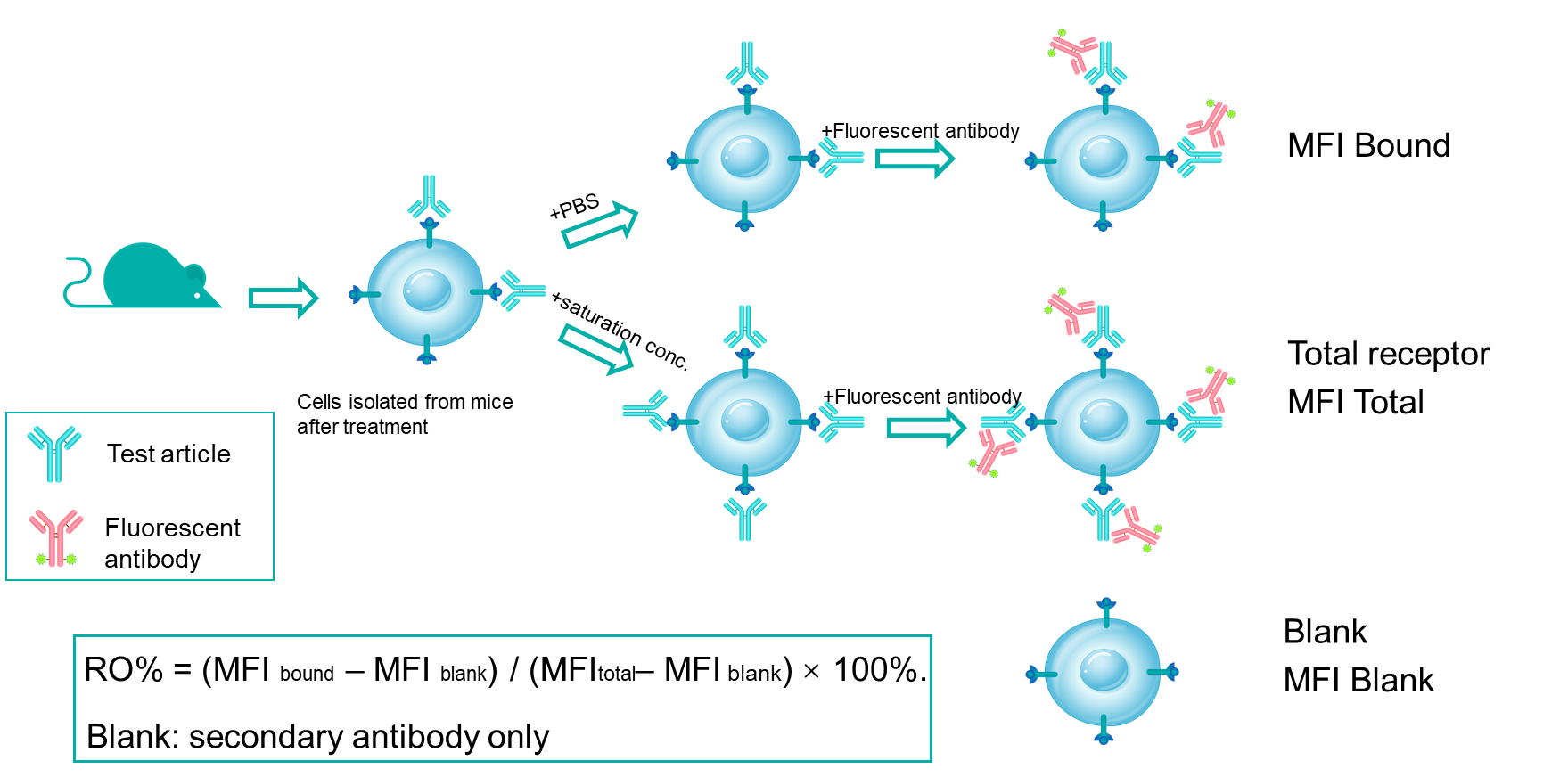
Mechanism of RO% Analysis
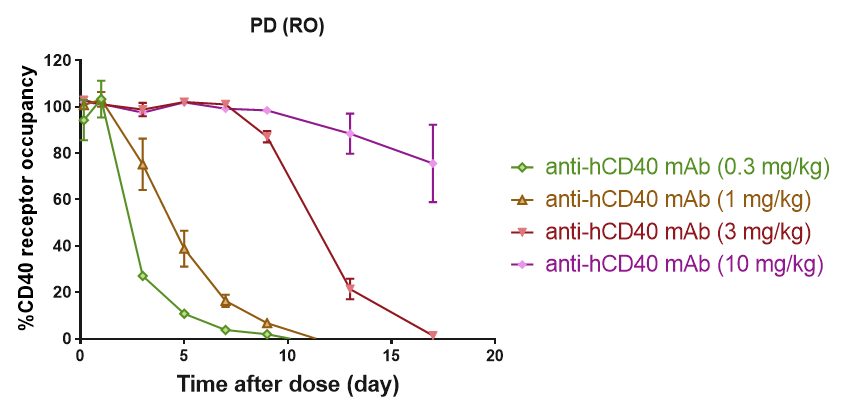
Receptor Occupancy Assay of CD40 Antibodies on CD19+ B Cells in Peripheral Blood of B-hCD40 Mice Humanized B-hCD40 mice were treated with different doses of an anti-human CD40 antibody, and the percentage of receptor occupancy on B cells of peripheral blood was assessed by flow cytometry.
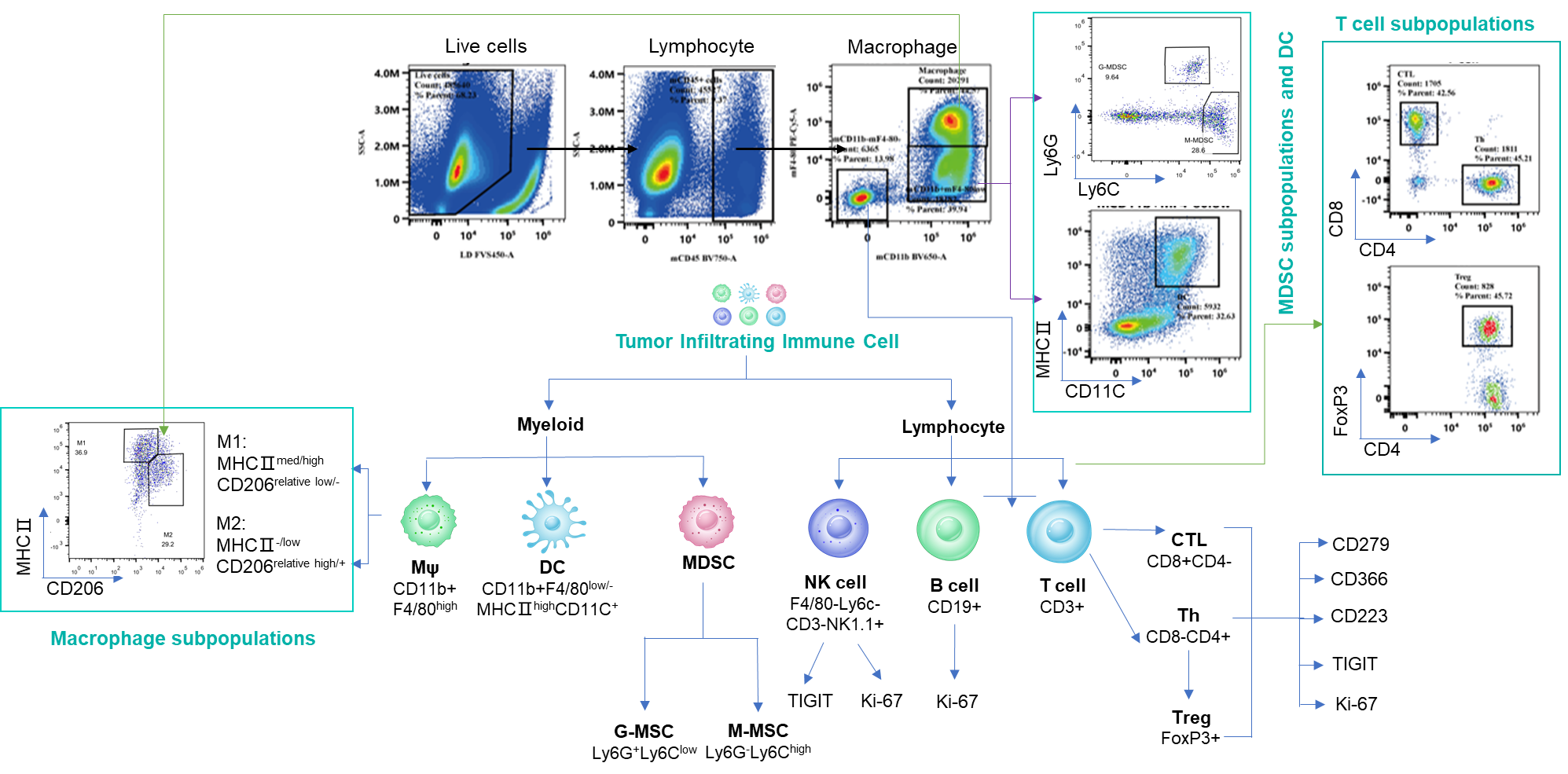
Analyzing tumor-infiltrating immune cells is essential for understanding the regulatory effects of drugs on the tumor immune microenvironment. Using multiparameter flow cytometry, we can comprehensively evaluate the infiltration levels and activation or inhibition states of various immune cell subsets within the tumor. For more detailed insights, we employ multiplex immunofluorescence and IHC studies to analyze immune cell infiltration and their spatial distribution within tumor tissue sections. These approaches provide critical data to elucidate the mechanisms of action of differentiated tumor immunotherapies.
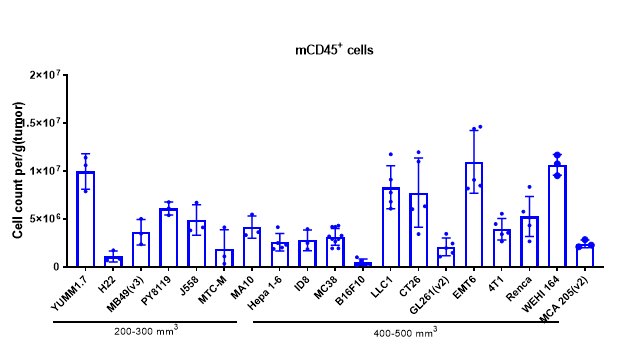
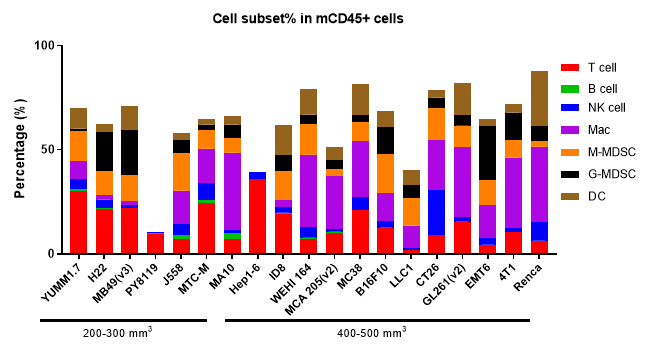
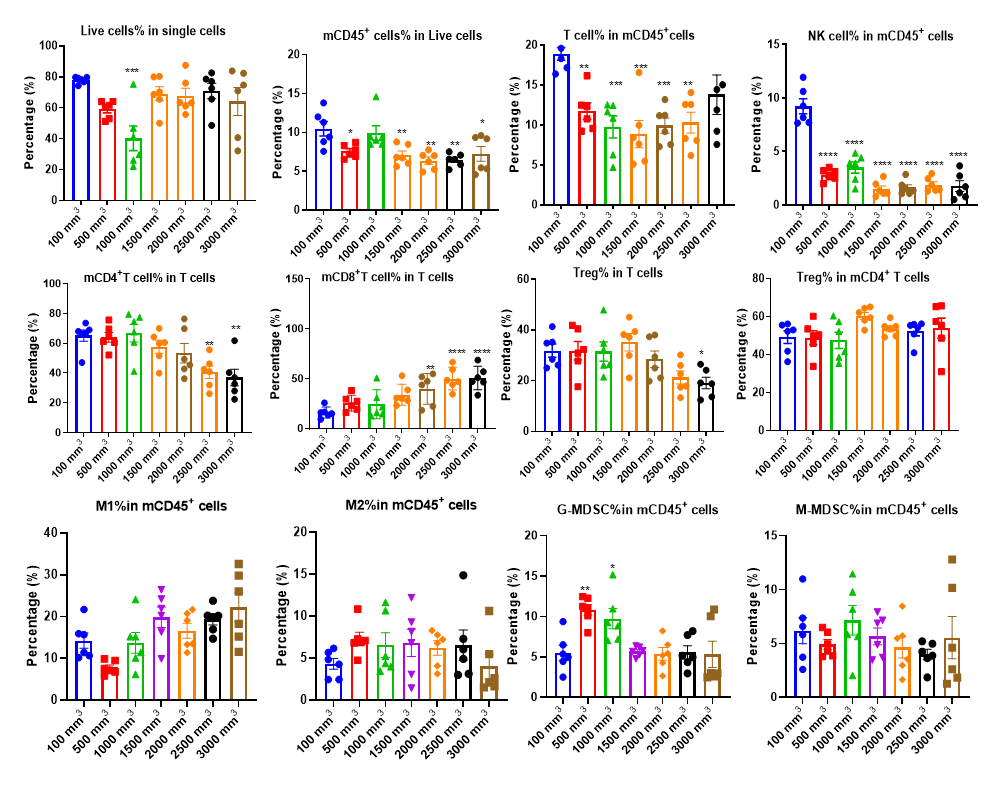
The Infiltration of Various Immune Cells in Different Volume of MC38 Tumor Data was shown as Mean±SEM, and analyzed using One way ANOVA followed Dunnett compared with100 mm3.
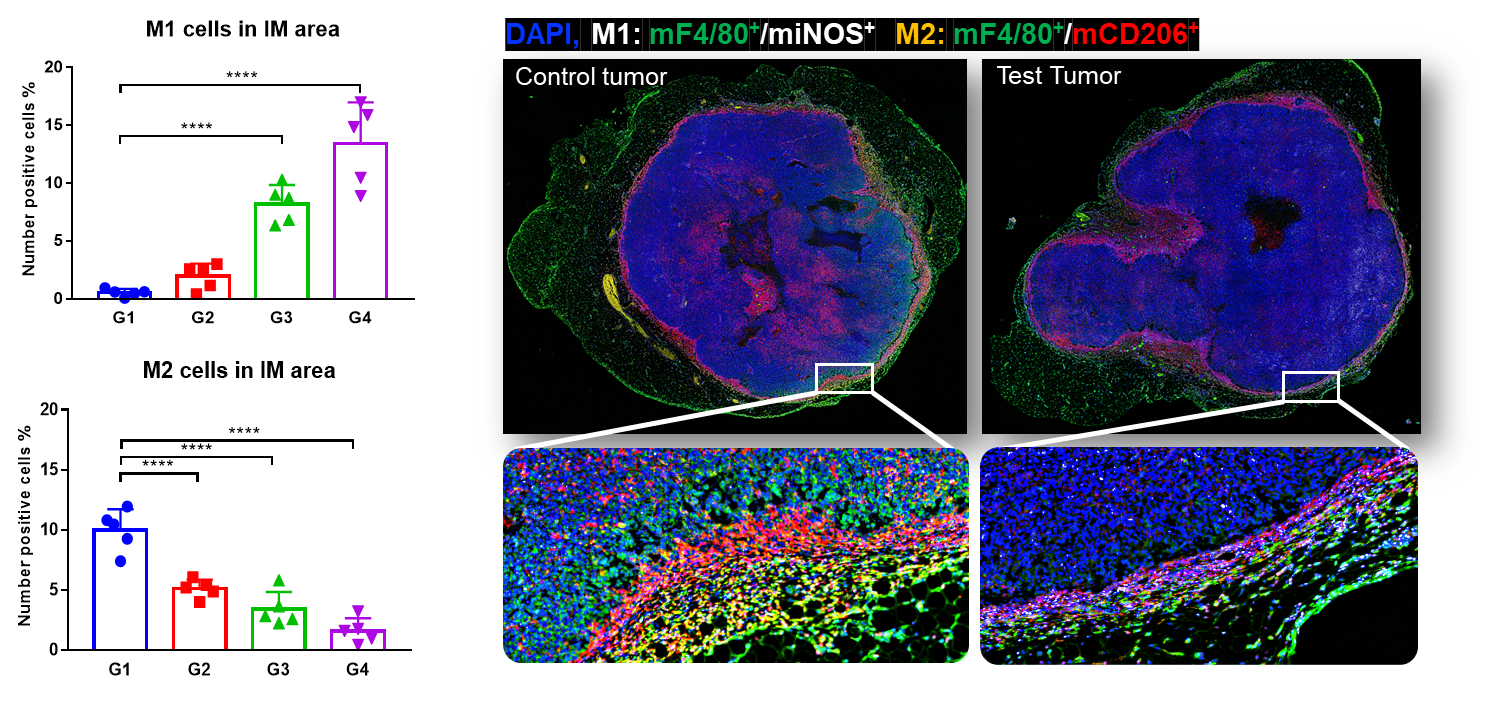
Quadro immunofluorescent full slide scanning of tumor tissues and co-localization of M1 and M2 in invasive margins. An increase of M1 was found in invasive margin of MC38 tumors, whereas M2 declined significantly in response to pembrolizumab treatment, with a dose-dependent manner. This indicates M1 infiltration facilitated anti-PD-1 mediated tumor growth inhibition.
TMA staining shows that CD8+ T (CTL) cells were increased dramatically in both tumor invasive margins and central tumor areas of Test. This is associated with an increased M1/M2 ratio.
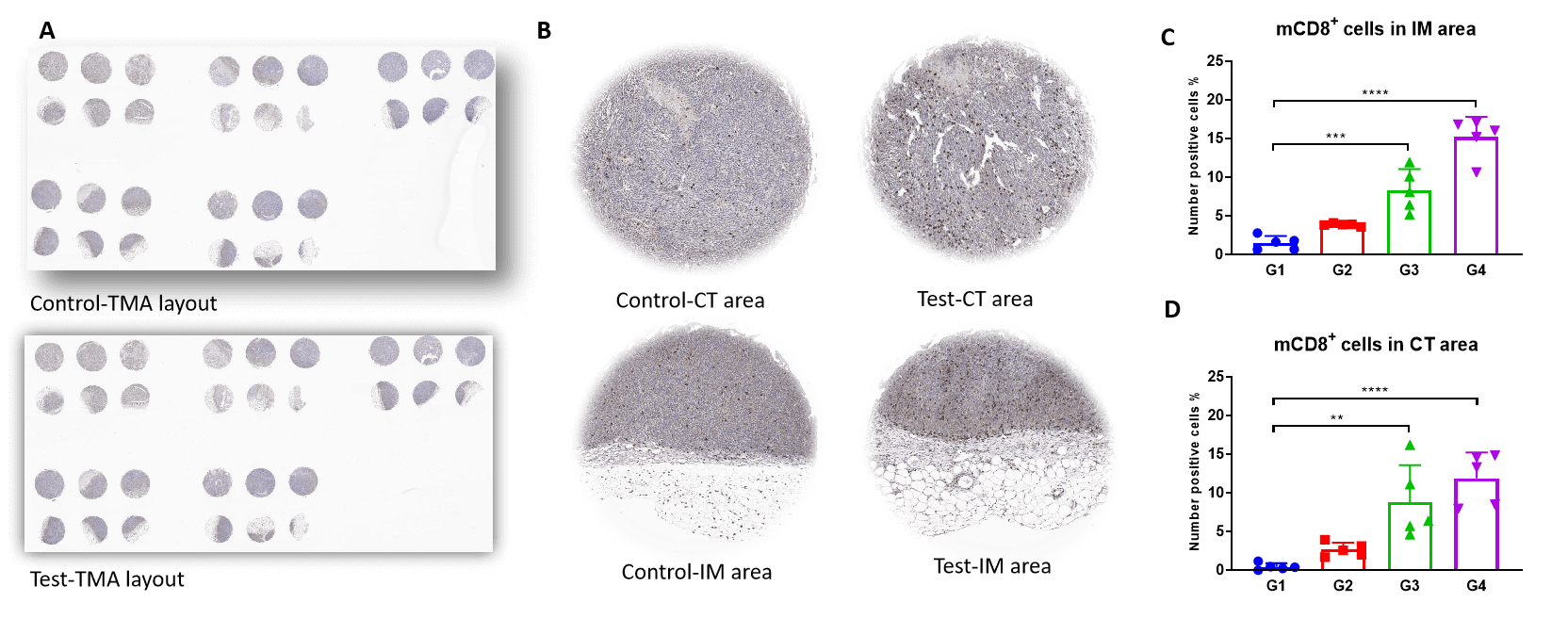
Tissue microarray IHC staining of mCD8+ T cells in central tumor and invasive margins
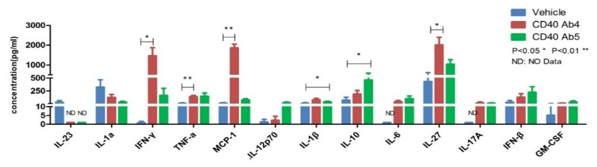
Cytokine analysis serves as a vital biomarker in the research of immunotherapy, immune-related diseases, and immune-related toxicities. We utilize ELISA, Luminex, and MSD platforms to conduct single or multiplex cytokine analysis on cell supernatants, serum, tissue fluids, and tissue homogenate samples. This supports pharmacological research in immuno-oncology and autoimmune diseases, as well as toxicological studies such as cytokine storm investigations.
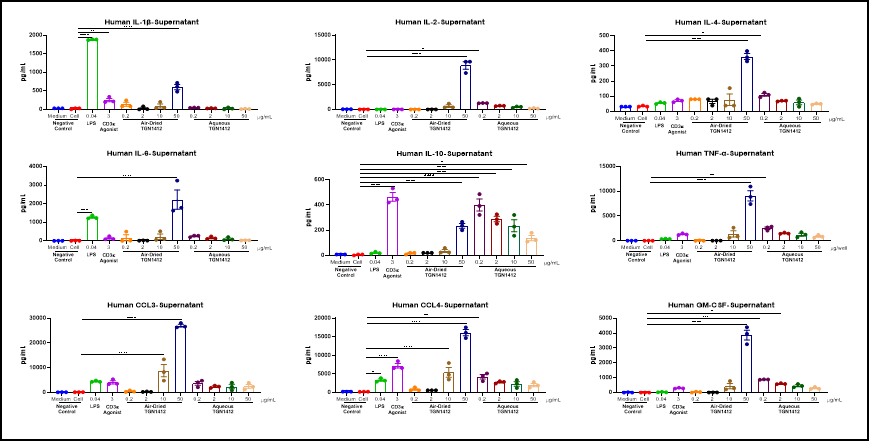
Air-dried TGN 1412 could induce inflammatory factor storm of PBMC in vitro
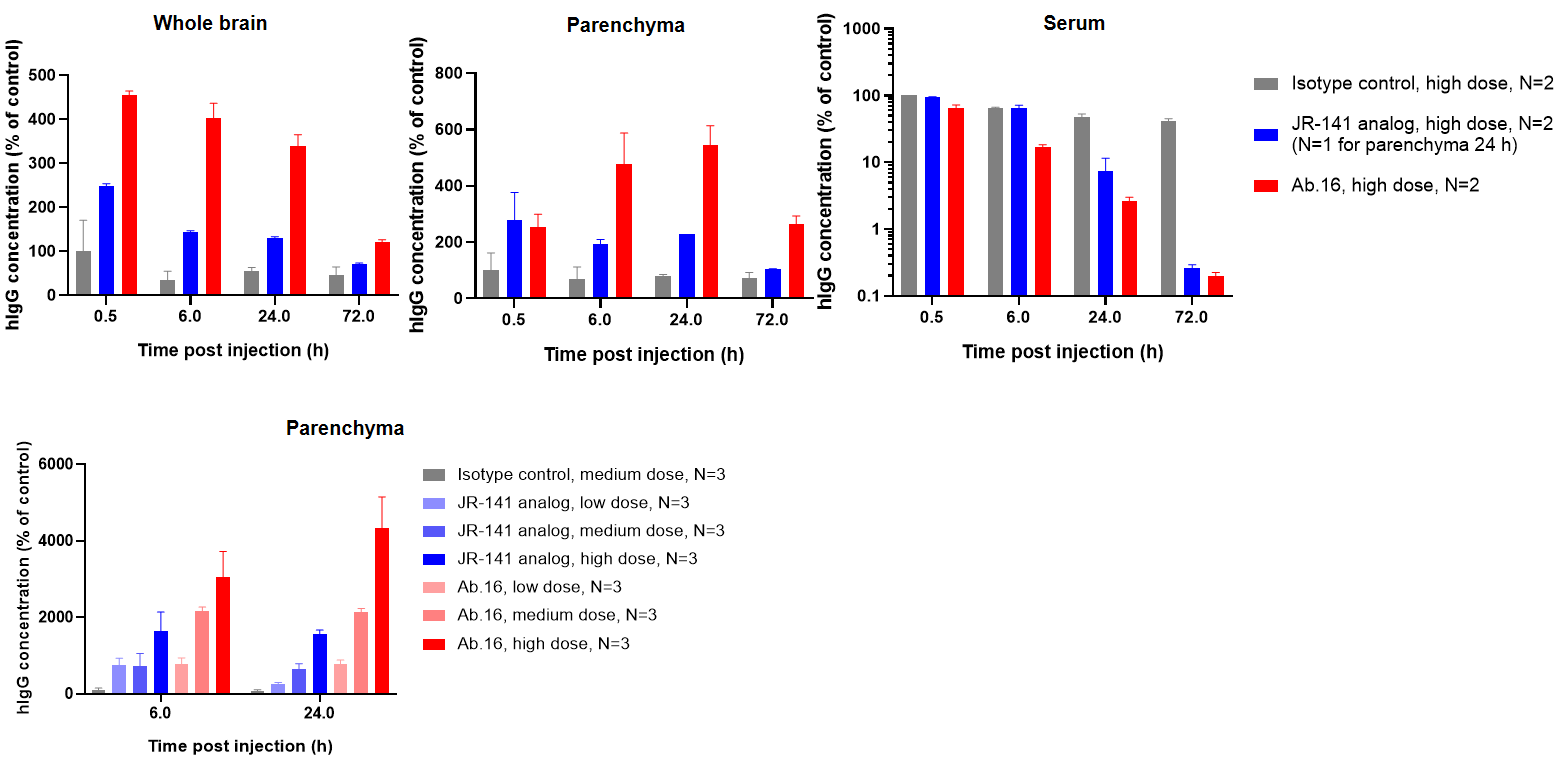
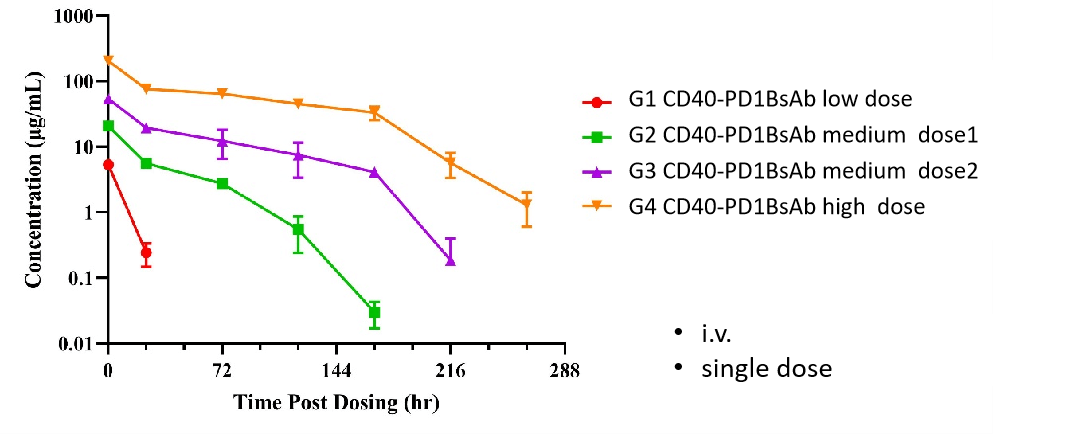
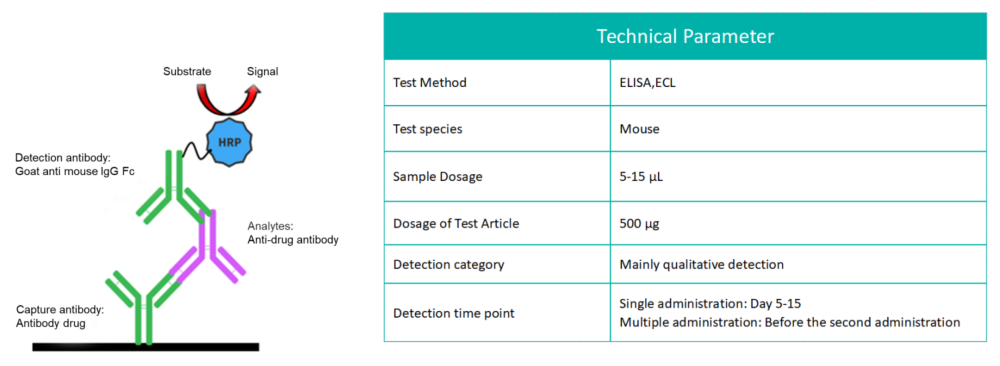
Biocytogen brings extensive experience in preclinical pharmacokinetic (PK) testing and bioanalysis, offering professional PK analysis services for antibody drugs. Our PK bioanalysis platform includes ELISA and Electrochemiluminescence (ECL) techniques.
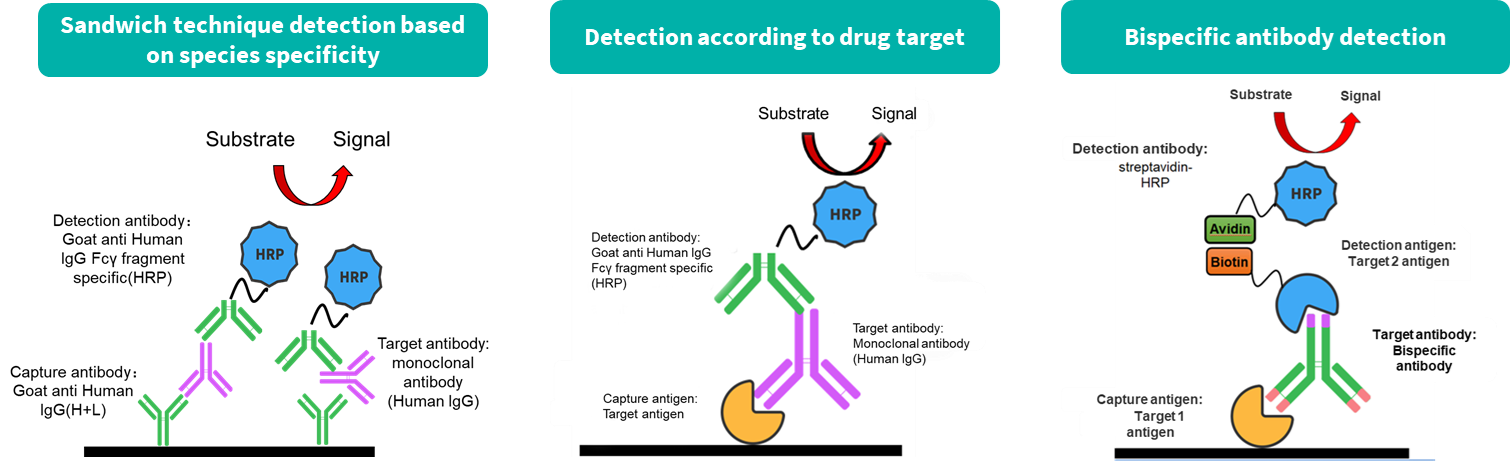
While antibody pharmacokinetic research differs from traditional chemical drug studies, it still relies on classical pharmacokinetic methods. However, greater attention is given to its targeting and the pharmacokinetic properties influenced by antibody characteristics. We offer two types of experimental services: antibody serum/plasma concentration detection and anti-drug antibody (ADA) screening.
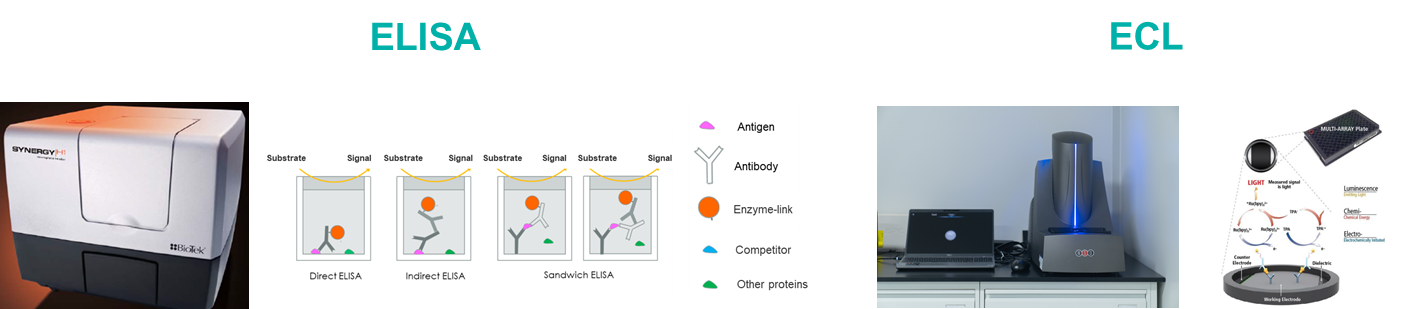
| ELISA | ECL | |
| Plates | Ordinary microporous plate | Graphite electrode microplate |
| Reaction Principle | Catalytic substrate luminescence after antigen antibody binding | Electrochemiluminescence after antibody antigen binding |
| Instrument | BioTek H1/EPOCH2 microplate reader | MSD |
| Advantage | Low cost; High flux; More experience | High sensitivity; High flux; Multi-factor detection; Low sample volume |
| Dynamic range | 2-3 logs | 3-5 logs |
| Purpose | Bioanalytical method validation; Quantitative detection; ADA | |
| Sample Type | Serum or plasma of mice, rats, dogs, monkeys, human, etc; Tumor; Brain tissue; Cerebrospinal fluid. | |
| Drug Type | Monoclonal antibody, Bispecific antibody, ADC, Fusion protein, etc. | |
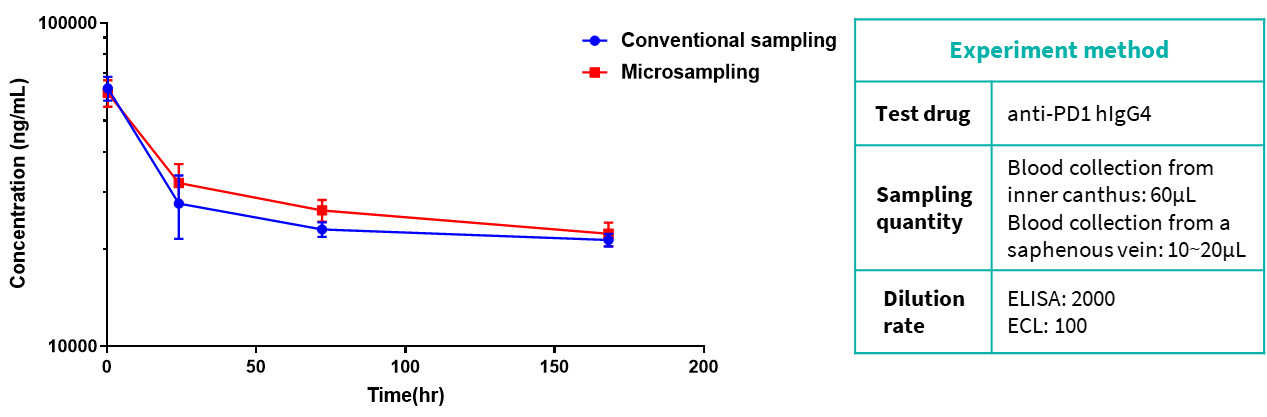
The data show that there is no obvious difference between the experimental results of the two sampling methods.
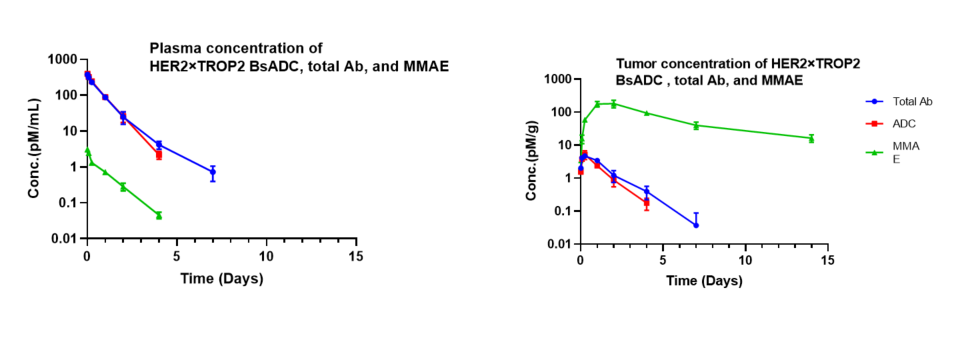
Pharmacokinetic analysis of HER2×TROP2 BsADC in NCI-H1975 xenograft model. After a single dose of 3 mg/kg of HER2×TROP2 BsADC , the payload MMAE was found to accumulate in the tumor but was present at low levels in the plasma. This suggests that HER2×TROP2 BsADC has reached an efficient tumor-targeted delivery of MMAE and may have better antitumor activity.
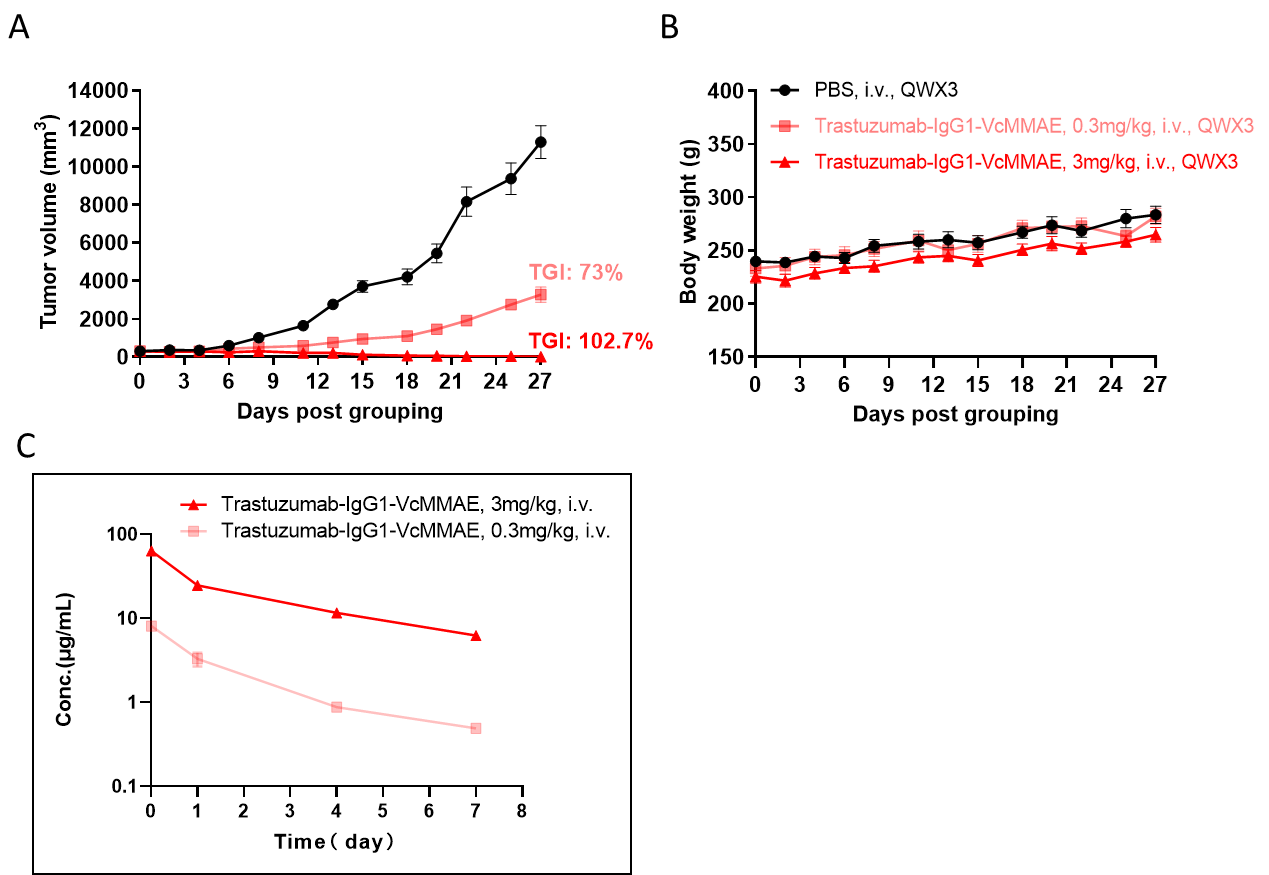
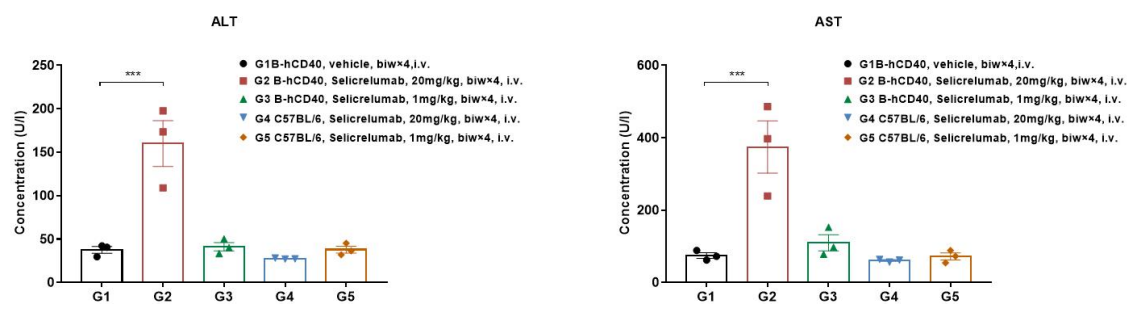
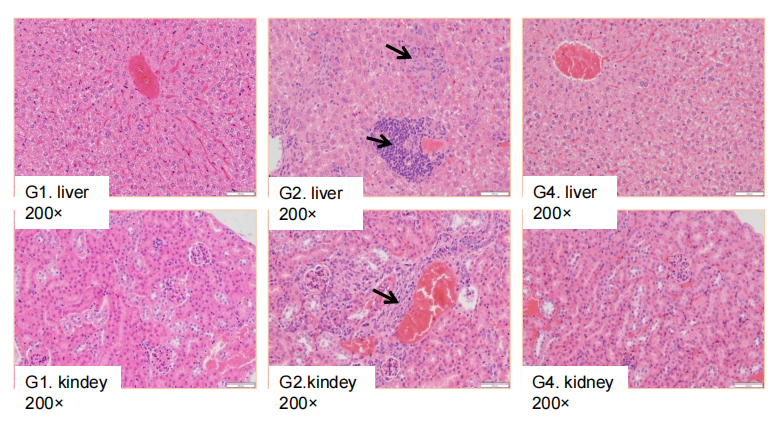
Antitumor activity of ADC in B-SDG rats. (A) ADC(Trastuzumab-IgG1-VcMMAE) inhibited NCI-H1975 tumor growth in B-SDG rats. Human non-small cell lung carcinoma cells (5E6) were subcutaneously implanted into B-SDG rats (female, 8-week-old, n=6). The rats were grouped when tumor volume reached approximately 300-400 mm3, at which time they were treated with ADC with doses indicated in panel. (B) Body weight changes during treatment. (C) PK test of ADC after last treatment. As shown in panel A, ADC was efficacious in controlling tumor growth in B-SDG rats, demonstrating that B-SDG rats provide a powerful preclinical model for in vivo evaluation of ADC. Values are expressed as mean ± SEM.
During treatment with biological products, some organisms may develop immune responses, with the most notable being the production of anti-drug antibodies (ADA). Immunogenicity refers to the ability of a substance to trigger an immune response. The formation of ADA-monoclonal antibody (mAb) immune complexes serves as an additional clearance pathway for monoclonal antibodies, significantly influencing their therapeutic effectiveness and elimination in the body.
The commonly used ADA detection method leverages the characteristics of the specific combination of ADA and the drug to detect ADA (essentially mouse anti-human IgG) produced in mice.
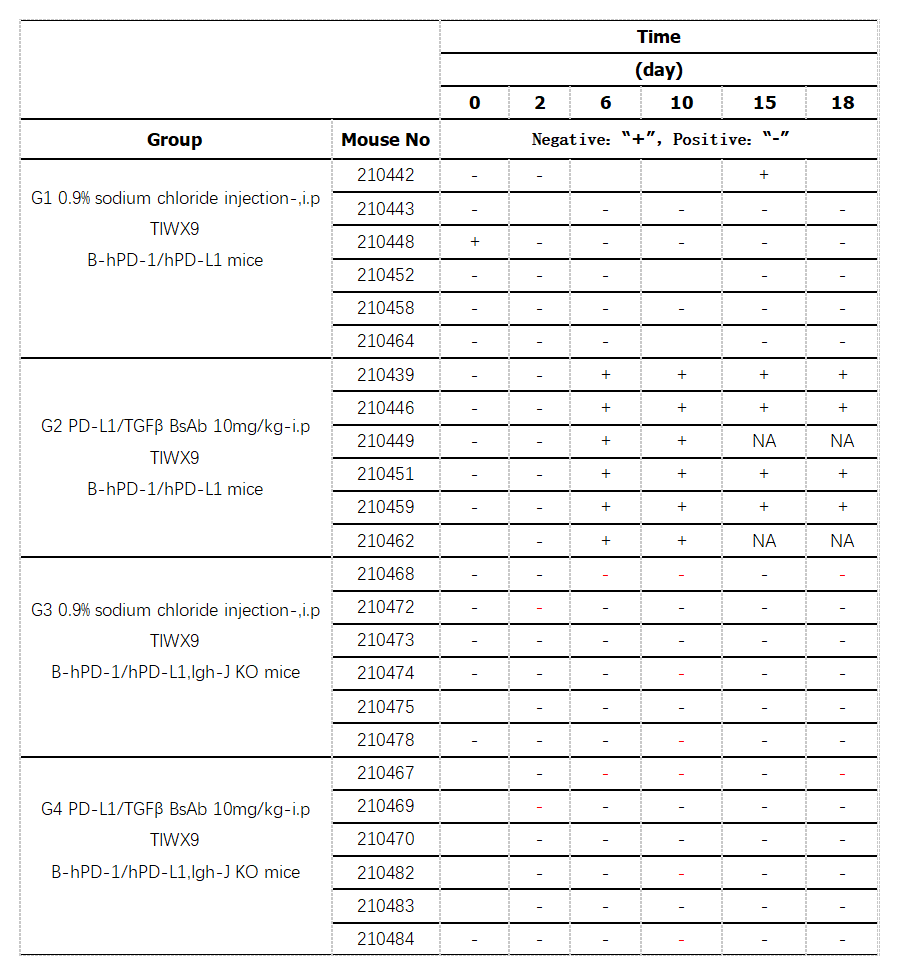
The SNR value (SNR= signal value of a single sample /pool series signal value) was calculated from the samples in the normal saline group and before administration. After excluding the abnormal value, the SNR of the remaining samples was used to calculate the screening threshold value of 1.24. The SNR values of all samples were compared with the screening threshold value, and those higher than the screening threshold value were recorded as positive "+" and those lower than the screening threshold value "-“. NA: unable to sample due to mouse death.