

on this page
Note: All MMAE-ADCs with a DAR of about 4
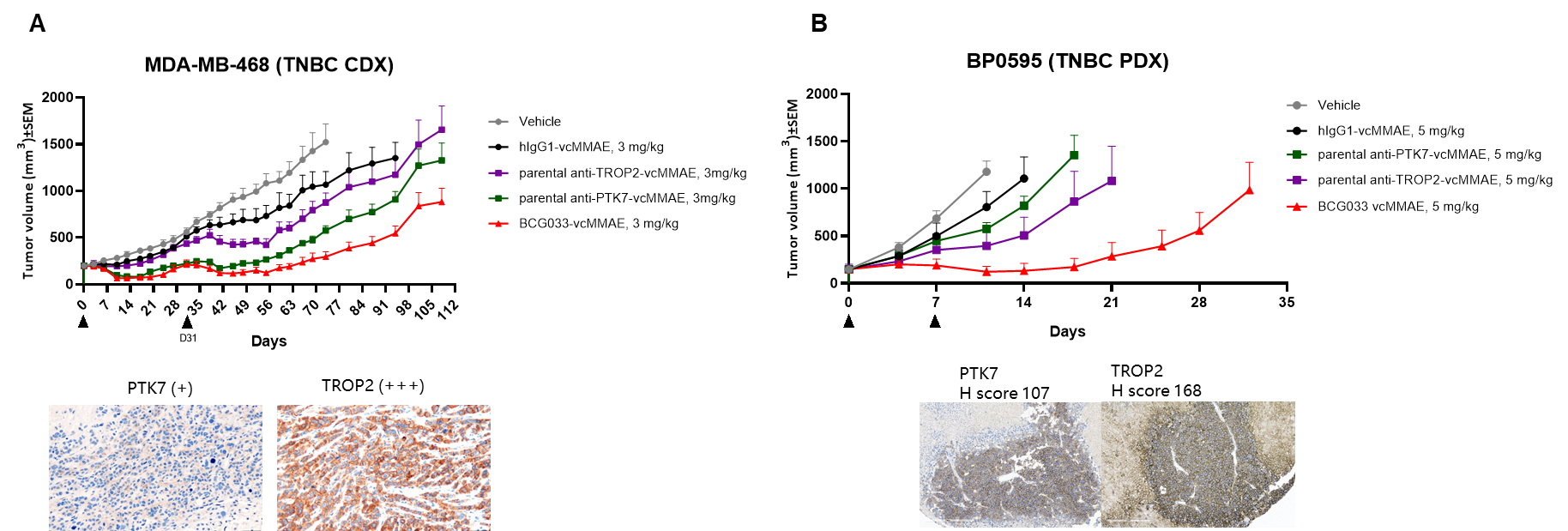
We first tested the anti-PTK7×TROP2 bsAb conjugate with vcMMAE (refer to BCG033-vcMMAE) for in vivo efficacy. The bsADC showed improved efficacy compared to the parental monoclonal Ab-ADCs in both TNBC CDX and PDX models, suggesting that bsADC has a synergistic effect and could overcome tumor heterogeneity.
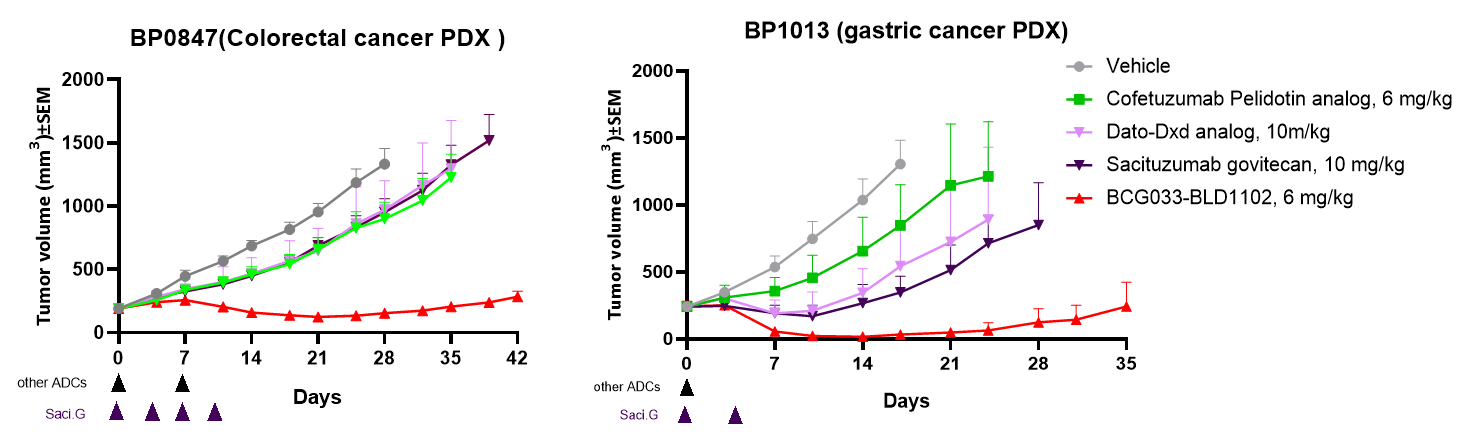
The BCG033 bsAb conjugated to BLD1102, a novel proprietary linker/payload composed of a DNA topoisomerase I inhibitor payload and a highly hydrophilic protease-cleavable linker, with a DAR of 8, are named BCG033-BLD1102.
BCG033-BLD1102 showed robust efficacy in two PDX models, outperforming benchmark ADCs.
Metastatic triple-negative breast cancer (TNBC) patients have poor overall survival, highlighting the need for novel treatments. Although the TROP2-targeting ADC sacituzumab govitecan recently received accelerated approval from the FDA for the treatment of patients with metastatic TNBC, the on-target toxicity of this single-target agent has limited clinical efficacy. We sought to improve the specificity and efficacy of future TNBC-targeted therapies by generating a bispecific antibody-drug conjugate (bsADC) targeting TROP2 and PTK7, another tumor-associated antigen (TAA) that is highly expressed in TNBC and correlates with poor prognosis and metastatic disease.
Learn more about the RenLite and ADC platform.