

• 322200

| Product name | B-hHER2 CT26.WT |
|---|---|
| Catalog number | 322200 |
| Strain background | BALB/c |
| Aliases | ERBB2, CD340, HER-2, HER-2/neu, HER2, MLN 19, NEU, NGL, TKR1, VSCN2, erb-b2 receptor tyrosine kinase 2 |
| Tissue | Colon |
| Disease | Colon carcinoma |
| Species | Mouse |
| Application | B-hHER2 CT26.WT cells have the capability to establish tumors in vivo |
on this page
Gene targeting strategy for B-hHER2 CT26.WT cells. The exogenous promoter and human HER2 coding sequence was inserted to replace part of murine exon 2 and all of exons 3-7. The insertion disrupts the endogenous murine Her2 gene, resulting in a non-functional transcript.
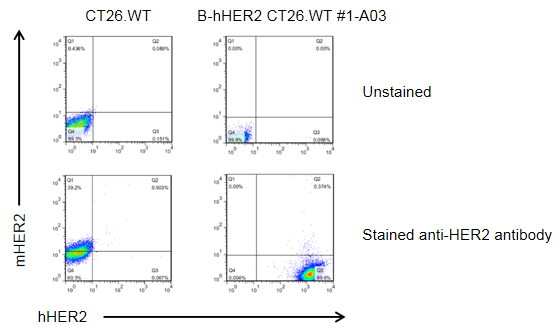
HER2 expression analysis in B-hHER2 CT26.WT cells by flow cytometry. Single cell suspensions from wild-type MC38 and B-hHER2 CT26.WT #1-A03 cultures were analyzed with anti-mouse HER2 antibody (RD, FAB6744P) and anti-human HER2 antibody (Biolegend, 324408) by flow cytometry. Human HER2 was detected on the surface of B-hHER2 CT26.WT cells but not wild-type MC38 cells.
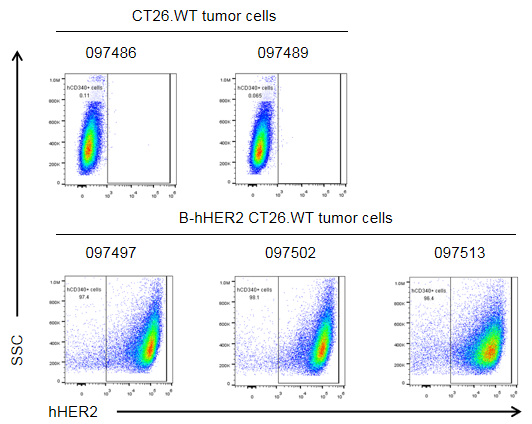
HER2 expression evaluated on B-hHER2 CT26.WT tumor cells by flow cytometry. B-hHER2 CT26.WT cells were subcutaneously transplanted into BALB/c mice (n=6). Upon conclusion of the experiment, tumor cells were harvested and analyzed with anti-human HER2 antibody (Biolegend, 324406) by flow cytometry. As shown, human HER2 was highly expressed on the surface of tumor cells. Therefore, B-hHER2 CT26.WT cells can be used for in vivo efficacy studies evaluating novel HER2 therapeutics.
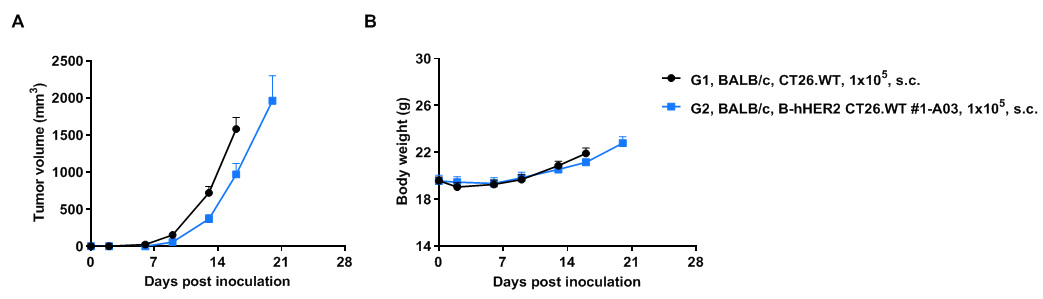
Subcutaneous tumor growth of B-hHER2 CT26.WT cells. B-hHER2 CT26.WT cells (1x105) and wild-type MC38 cells (1x105) were subcutaneously implanted into BALB/c mice (female, 6-7-week-old, n=6). Tumor volume and body weight were measured twice a week. (A) Average tumor volume. (B) Body weight. Volume was expressed in mm3 using the formula: V=0.5 X long diameter X short diameter2. Results indicate that B-hHER2 CT26.WT cells were able to establish tumors in vivo and can be used for efficacy studies. Values are expressed as mean ± SEM.
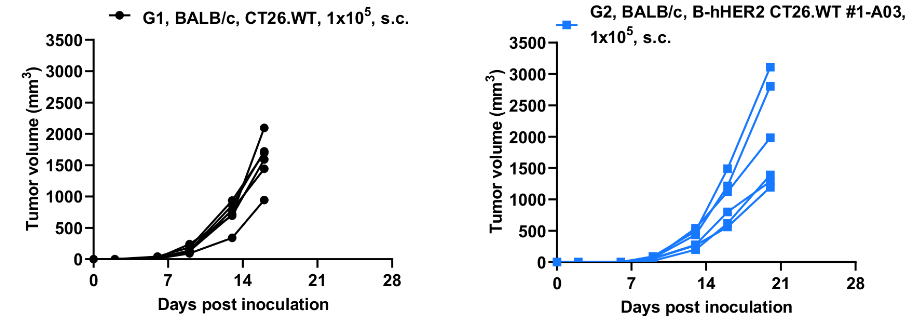
B-hHER2 CT26.WT tumor growth curves from individual mice. B-hHER2 CT26.WT cells (1x105) and wild-type MC38 cells (1x105) were subcutaneously implanted into BALB/c mice (female, 6-7-week-old, n=6). Results indicate that B-hHER2 CT26.WT cells were able to establish tumors in vivo and can be used for efficacy studies. Values are expressed as mean ± SEM.
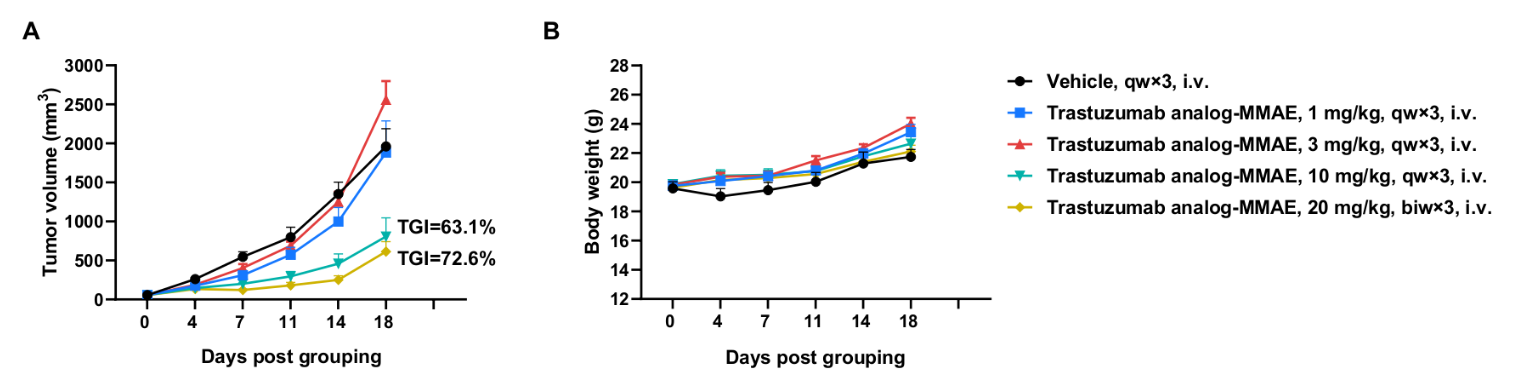
Antitumor activity of anti-human HER2 ADC (Trastuzumab analog-MMAE, in-house) in BALB/c mice. (A) Anti-human HER2 ADC inhibited B-hHER2 CT26.WT tumor growth in BALB/c mice. Murine colon cancer B-hHER2 CT26.WT cells were subcutaneously implanted into BALB/c mice (female, 9-week-old, n=6). Mice were grouped when tumor volume reached approximately 50 mm3, at which time they were intraperitoneally injected with anti-human HER2 ADC Trastuzumab analog-MMAE (in-house) indicated in panel. (B) Body weight changes during treatment. As shown in panel A, anti-human HER2 ADC was efficacious in controlling tumor growth in BALB/c mice, demonstrating that B-hHER2 CT26.WT provide a powerful preclinical model for in vivo evaluation of anti-human HER2 antibodies. Values are expressed as mean ± SEM.
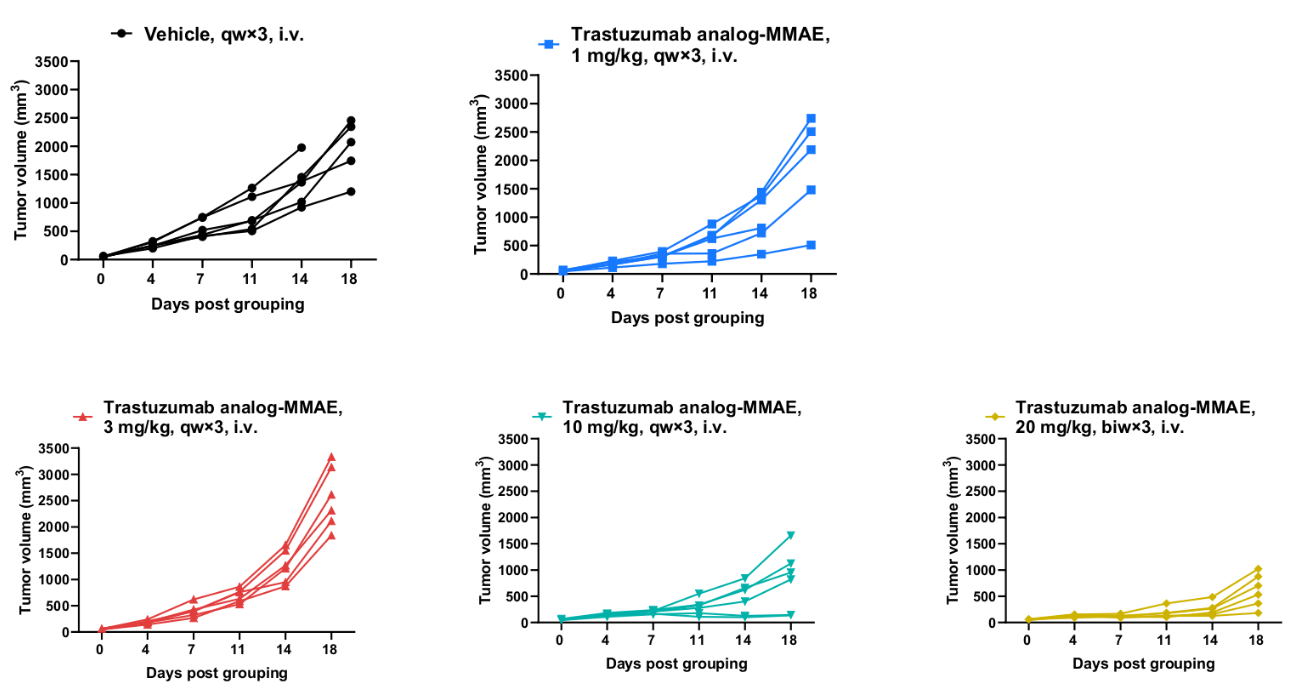
Antitumor activity of anti-human HER2 ADC (Trastuzumab analog-MMAE, in-house) in BALB/c mice. Murine colon cancer B-hHER2 CT26.WT cells were subcutaneously implanted into BALB/c mice (female, 9-week-old, n=6). Mice were grouped when tumor volume reached approximately 50 mm3, at which time they were intraperitoneally injected with anti-human HER2 ADC Trastuzumab analog-MMAE (in-house) indicated in panel.