


C57BL/6N-Cd38tm3(CD38)Bcgen/Bcgen • 110046
| Product name | B-hCD38 mice |
|---|---|
| Catalog number | 110046 |
| Strain name | C57BL/6N-Cd38tm3(CD38)Bcgen/Bcgen |
| Strain background | C57BL/6N |
| Aliases | CD38, ADPRC 1, ADPRC1 |
on this page
CD38 is a 42 kD glycoprotein, also known as T10. It is an ADP-ribosyl hydrolase, expressed on B cells, NK cells, a subset of T cells, brain, muscle, and kidney. In mouse, CD38 expression is downregulated on germinal center B cells and plasma cells, whereas this is not the case for humans. By functioning as both a cyclase and a hydrolase, CD38 mediates lymphocyte activation, as well as adhesion and metabolism of cADPR and NAADP. CD31 is also the ligand of CD38. NAD+ is disassembled into byproducts that flow in the BM plasma fluid within the myeloma niche, accumulating variable amounts of ADO. Most of ADO is taken-up by purinergic cell receptors (ADOR) expressed by bone cells or immune cells inside the niche. The outcome is either a block of the effectiveness of immune cells (Teff, NK, TAMs) that are capable of destroying tumor cells or that increase the number of regulatory T-cells (Tregs), mesenchymal derived stromal cells (MDSC), or dendritic cells (DC) which suppress immune cells from responding to the tumor. Increased expression of CD38 is an unfavorable diagnostic marker in chronic lymphocytic leukemia and is associated with increased disease progression. CD38 is also used as a target for daratumumab (Darzalex), a medicine that has been approved for the treatment of multiple myeloma.
Gene targeting strategy for B-hCD38 mice. The CDS of human CD38 encoding the extracellular domain was inserted following partial exon 2 of mouse Cd38 in B-hCD38 mice.

Strain specific analysis of CD38 gene expression in WT and hCD38 mice by RT-PCR. Mouse Cd38 mRNA was detectable only in splenocytes of wild-type (+/+) mice. Human CD38 mRNA was detectable only in H/H, but not in +/+ mice.
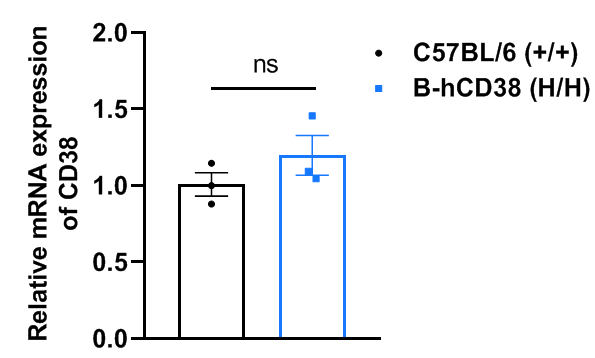
Strain specific analysis of CD38 gene expression in wild-type C57BL/6 mice and B-hCD38 mice by RT-qPCR. The mRNA expression of CD38 in homozygous B-hCD38 mice (H/H) was similar to those in the wild-type C57BL/6 mice (+/+), demonstrating that introduction of hCD38 in place of its mouse counterpart does not change the CD38 expression. Values are expressed as mean ± SEM. Significance was determined by unpaired t-test. *P < 0.05, **P < 0.01, ***P < 0.001.
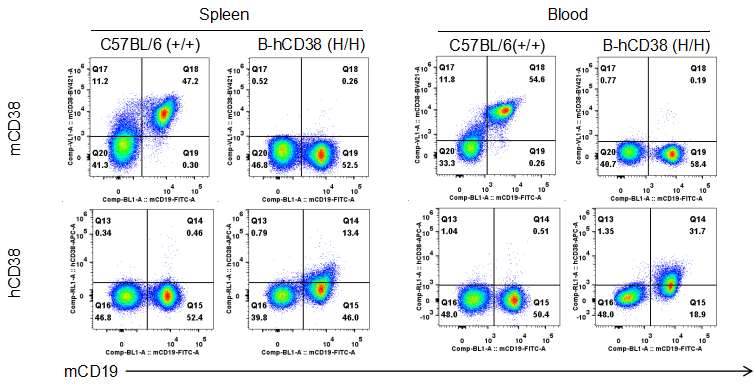
Strain specific CD38 expression analysis in homozygous B-hCD38 mice by flow cytometry. Splenocytes and blood were collected from WT and homozygous B-hCD38 mice(H/H) , and analyzed by flow cytometry with species-specific anti-CD38 antibody. Mouse CD38 was detectable in WT mice. Human CD38 was exclusively detectable in homozygous B-hCD38 but not WT mice.
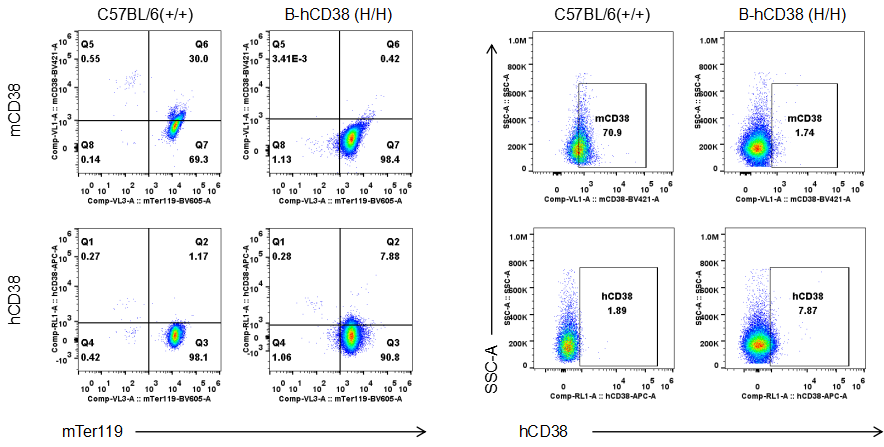
Strain specific CD38 expression analysis in homozygous B-hCD38 mice by flow cytometry. Blood were collected from WT and homozygous B-hCD38 mice(H/H) , and analyzed by flow cytometry with species-specific anti-CD38 antibody. Mouse CD38 was detectable in WT mice. Human CD38 was exclusively detectable in homozygous B-hCD38 mice but not WT mice.
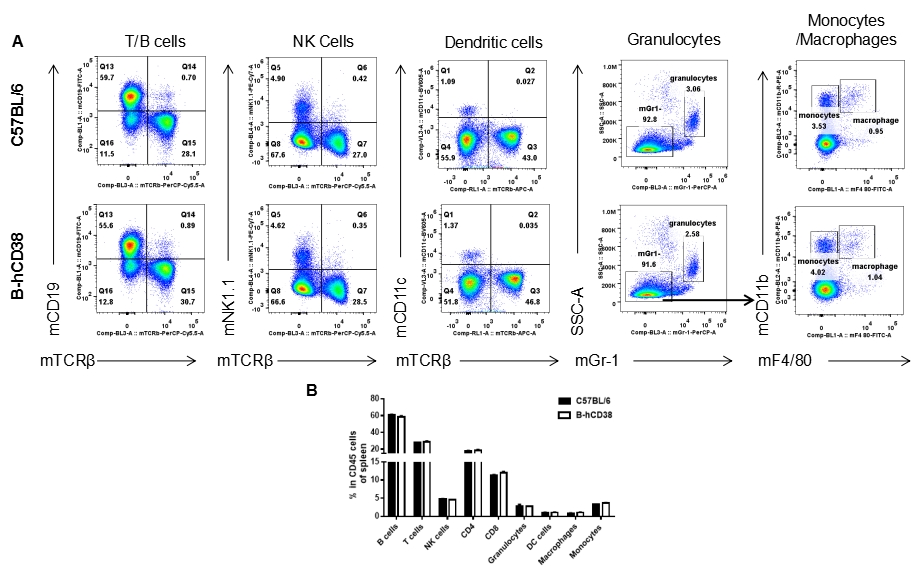
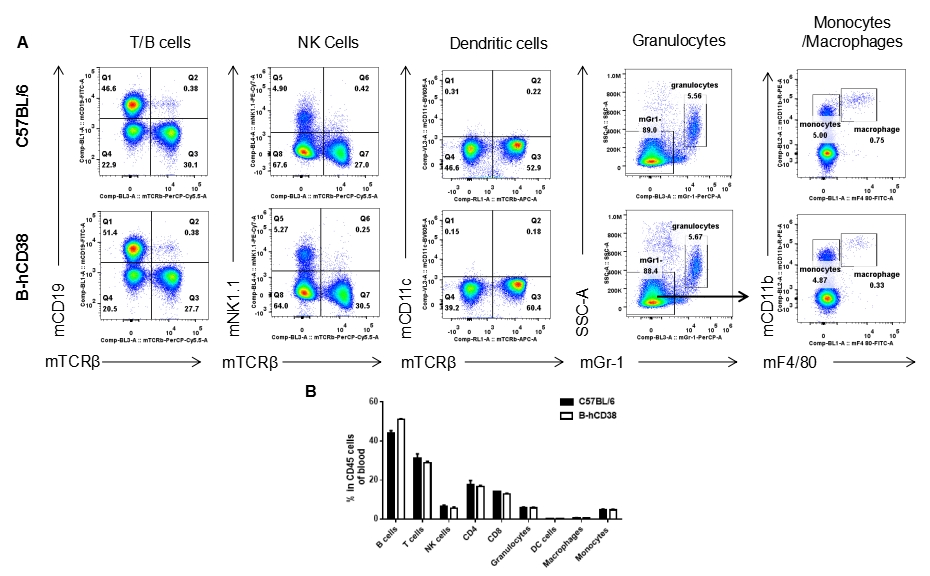
Analysis of splenic leukocyte subpopulations by FACS. Splenocytes were isolated from female C57BL/6 and B-hCD38 mice (n=3, 6 weeks-old) and analyzed by flow cytometry to assess leukocyte subpopulations. (A) Representative FACS plots gated on single live CD45+ cells for further analysis. (B) Results of FACS analysis. Percentages of T, B, NK cells, monocytes/macrophages, and DC were similar in homozygous B-hCD38 mice and C57BL/6 mice, demonstrating that introduction of hCD38 in place of its mouse counterpart does not change the overall development, differentiation, or distribution of these cell types in spleen. Values are expressed as mean ± SEM.
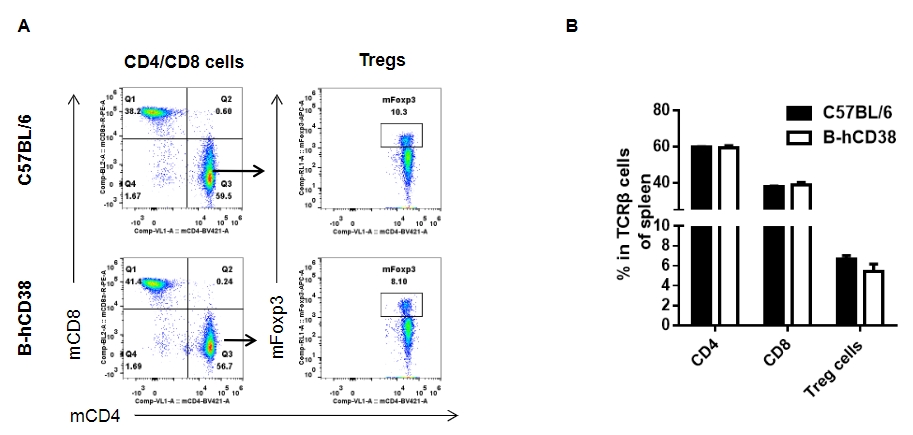
Analysis of splenic T cell subpopulations by FACS. Splenocytes were isolated from female C57BL/6 and B-hCD38 mice (n=3, 6 weeks-old) and analyzed by flow cytometry for T cell subsets. (A) Representative FACS plots gated on TCRβ+ T cells and further analyzed. (B) Results of FACS analysis. Percentages of CD8+ T cells, CD4+ T cells and Tregs were similar in homozygous B-hCD38 and C57BL/6 mice, demonstrating that introduction of hCD38 in place of its mouse counterpart does not change the overall development, differentiation or distribution of these T cell subtypes in spleen. Values are expressed as mean ± SEM.
Analysis of splenic T cell subpopulations by FACS. Splenocytes were isolated from female C57BL/6 and B-hCD38 mice (n=3, 6 weeks-old) and analyzed by flow cytometry for T cell subsets. (A) Representative FACS plots gated on TCRβ+ T cells and further analyzed. (B) Results of FACS analysis. Percentages of CD8+ T cells, CD4+ T cells and Tregs were similar in homozygous B-hCD38 and C57BL/6 mice, demonstrating that introduction of hCD38 in place of its mouse counterpart does not change the overall development, differentiation or distribution of these T cell subtypes in spleen. Values are expressed as mean ± SEM.
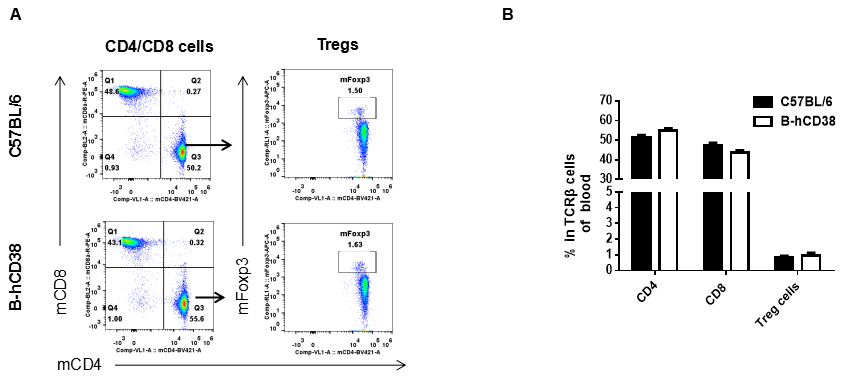
Analysis of blood T cell subpopulations by FACS. Blood were isolated from female C57BL/6 and B-hCD38 mice (n=3, 6 weeks-old) and analyzed by flow cytometry for T cell subsets. (A) Representative FACS plots gated on TCRβ+ T cells and further analyzed. (B) Results of FACS analysis. Percentages of CD8+ T cells, CD4+ T cells and Tregs were similar in homozygous B-hCD38 and C57BL/6 mice, demonstrating that introduction of hCD38 in place of its mouse counterpart does not change the overall development, differentiation or distribution of these T cell subtypes in blood. Values are expressed as mean ± SEM.
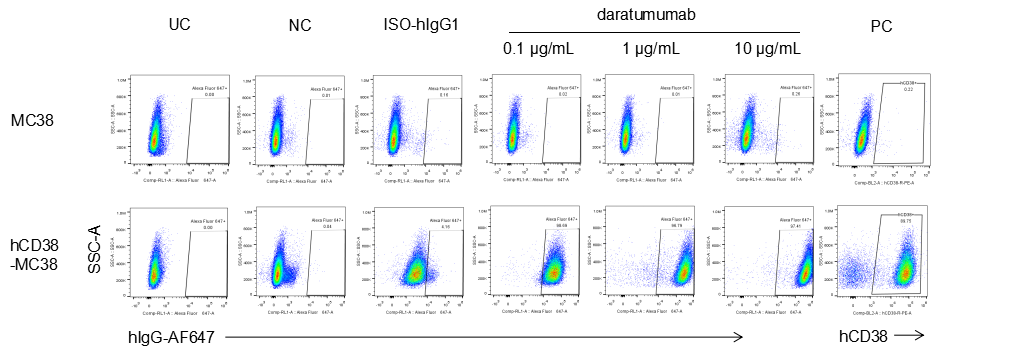
Analysis of B-CAG-hCD38 MC38 by FACS. Flow cytometry analysis of the B-CAG-hCD38 MC38 was performed to assess anti-human CD38 Ab binding. B-CAG-hCD38 MC38 can bind well to anti-hCD38 antibody (daratumumab, in house) vs isotype control.
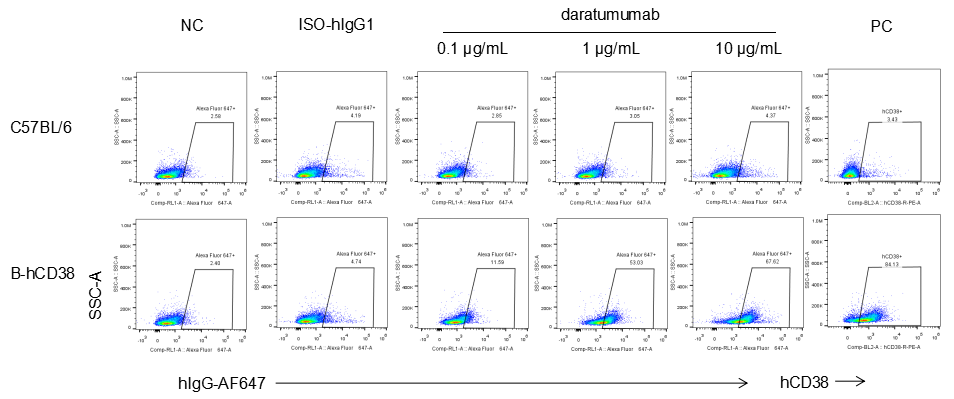
Analysis of splenocytes of B-hCD38 mice by FACS. Splenocytes were isolated from female B-hCD38 mice (6 week-old). Flow cytometry analysis of the splenocytes was performed to assess anti-human CD38 Ab binding with splenocytes. Single live cells were gated for CD19+ population and used for further analysis as indicated here. Splenocytes in homozygous B-hCD38 mice can bind well to anti-hCD38 antibody (daratumumab, in house) vs isotype control.
Panel 1: mTER-119, G-anti-hIgG-AF647
Panel 2: Live/Dead, mCD45, mCD3, mCD4, mCD8a, mNK1.1, mFoxp3, G-anti-hIgG-AF647
Panel 3: Live/Dead, mCD45, mCD11b, mCD14, mF4/80, G-anti-hIgG-AF647
Panel 4: Live/Dead, mCD45, mCD3, mCD4, mCD8a, mNK1.1, mFoxp3, hCD38-APC
Panel 5: Live/Dead, mCD45, mCD11b, mCD14, mF4/80, hCD38-APC
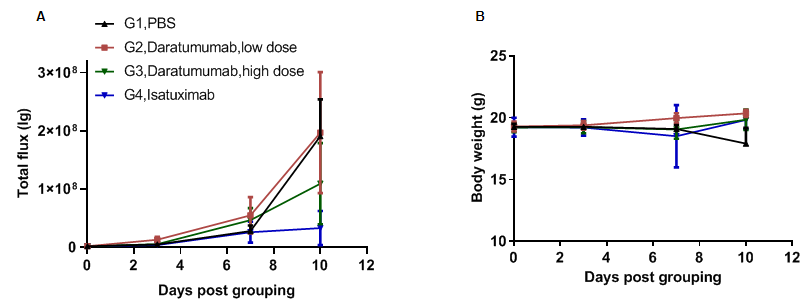
Anti-tumor activity of anti-human CD38 antibody in B-hCD38 mice. (A) Anti-human CD38 antibody (in house) inhibit B-hCD38-luc E.G7-OVA tumor growth in B-hCD38 mice. Murine T lymphocytoma B-hCD38-luc E.G7-OVA cells were injected by tail vein into homozygous B-hCD38 mice (female, 6 week-old, n=6). Mice were grouped when total flux reached approximately 106 Ig, at which time they were treated with anti-human CD38 antibodies indicated in panel. (B) Body weight changes during treatment. As shown in panel A, anti-human CD38 antibodies were efficacious in controlling tumor growth. Values are expressed as mean ± SEM.
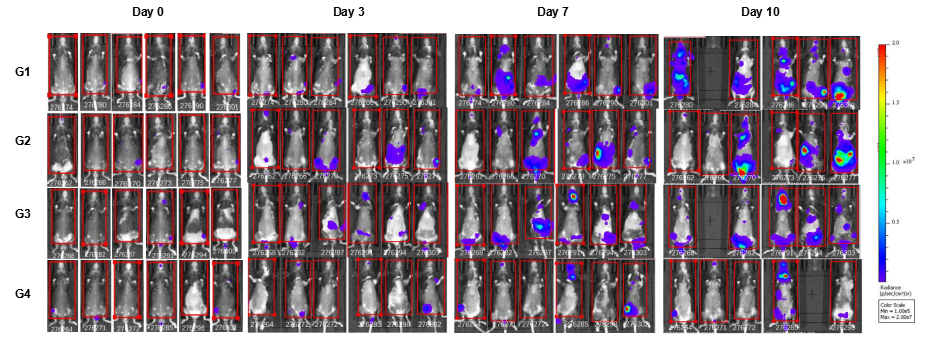
In vivo luciferase images of B-hCD38-Luc E.G7-OVA cells. Mice were grouped when total flux reached approximately 106 Ig, at which time they were treated with anti-human CD38 antibodies. Signal intensity and body weight were measured twice a week. Imaging was performed on days 0, 3, 7 and 10. Values are expressed as mean ± SEM.
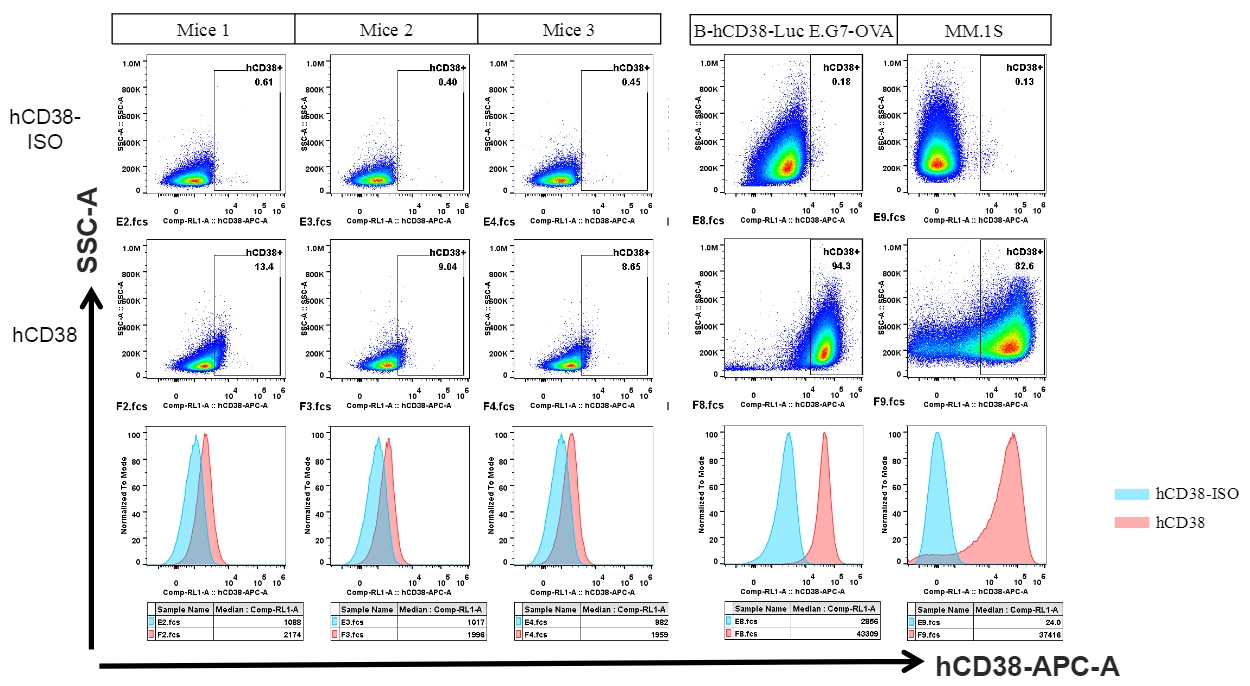
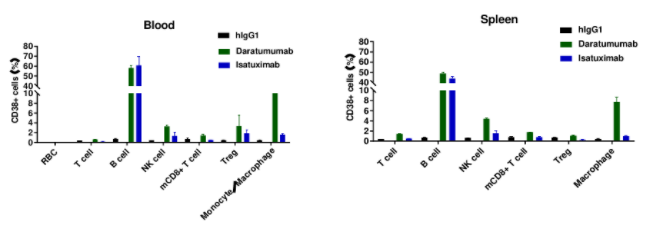
Human CD38 was detected in B cells of spleen from B-hCD38 mice and cell lines.
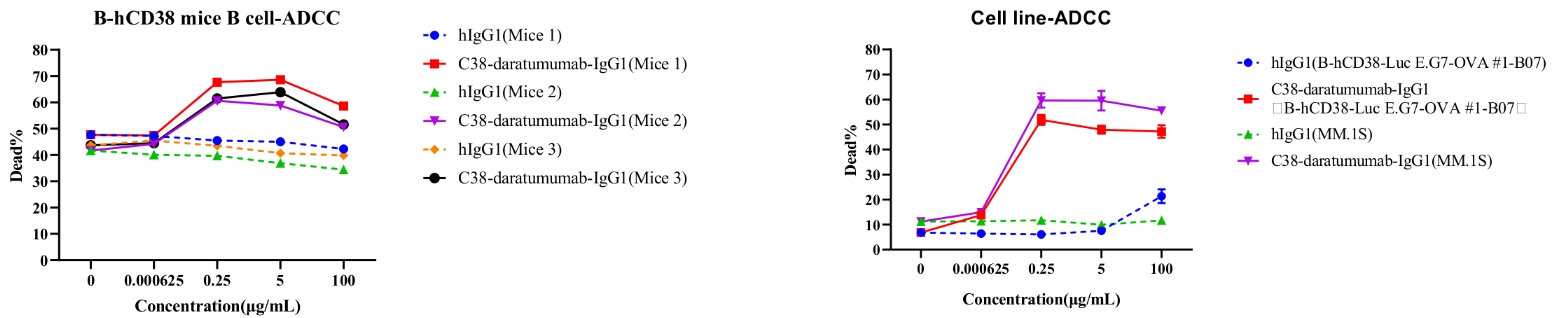
Daratumumab-mediated ADCC against B cells of spleen in B-hCD38 mice.
ADCC assays against B cells with FcR-TANK cells as effector cells. Antibodies were added to cells at several concentrations shown in the panel. FcR-TANK cells were added to target cells at an ET ratio of 5:1. After 4 h at 37℃, flow cytometry was performed to assess specific lysis. Results of three different mice are shown (left panel).
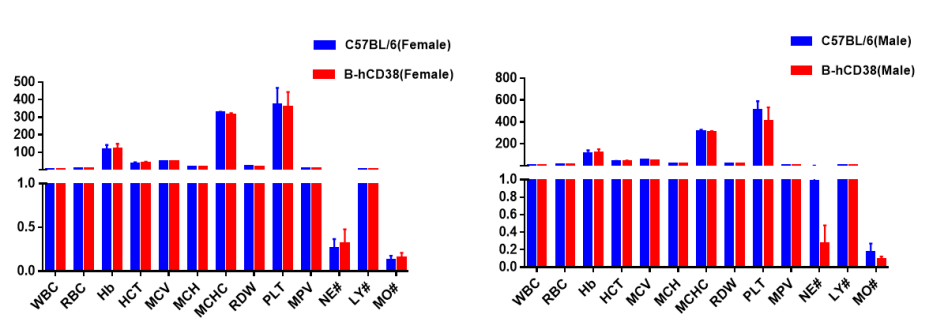
Complete blood count (CBC). Blood from C57BL/6 and B-hCD38 mice (n=5, 6 week-old, female and male) were collected and analyzed for CBC. Any measurement of B-hCD38 mice in the panel were similar to C57BL/6, and there was no differences between male and female mice, indicating that humanized mouse does not change blood cell composition and morphology. Values are expressed as mean ± SEM.
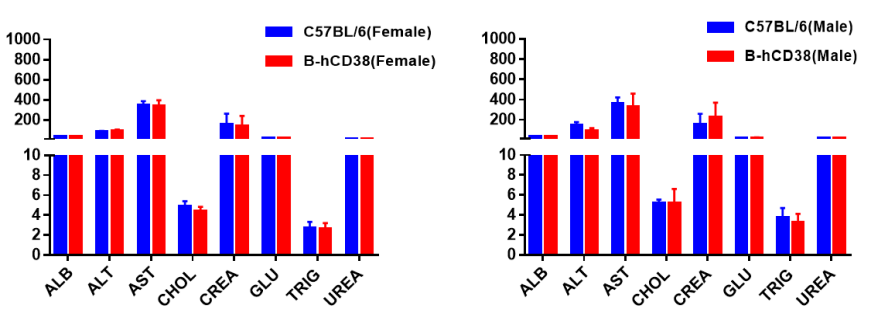
Blood chemistry tests of B-hCD38 mice. Serum from C57BL/6 and B-hCD38 mice (n=5, 6 week-old, female and male) were collected and analyzed for levels of ALT, AST and other indicators in the panel. There was no differences on either measurement between C57BL/6 and humanized mouse, indicating that humanized mouse does not change ALT and AST levels or health of liver. Values are expressed as mean ± SEM.