


NOD.CB17-Prkdcscid Il2rgtm1/Bcgen • 112469
| Product name | huPBMC-B-NDG mice |
|---|---|
| Catalog number | 112469 |
| Strain name | NOD.CB17-Prkdcscid Il2rgtm1/Bcgen |
| Strain background | NOD scid |
| Aliases | Prkdc: DNA-PKcs, DNAPDcs, DNAPK, DNPK1, DOXNPH, HYRC1, XRCC7, dxnph, p460, scid, slipIl2rg: CD132, [g]c, gamma(c), gc, p64 |
| Application | Human T cell reconstitution;Human-derived cell or tissue engraftment;Tumor researchl Human disease model study;Efficacy and toxicity verification for human targeted drugs |
The immunodeficient B-NDG mice (NOD.CB17-PrkdcscidIl2rgtm1/Bcgen) was independently designed and generated by Biocytogen. B-NDG mice were generated by deleting the Il2rg gene from NOD scid mice with severe immunodeficient phenotype. This mouse model lacks mature T cells, B cells and functional NK cells. It is internationally recognized as an immunodeficient mouse model well suited for human-derived tissue or cell engraftment.
B-NDG mice: Combined NOD scid-Il2rg null background features, severe immunodeficient phenotype, absence of mature T cells, B cells, and functional NK cells, decreased function of macrophages and dendritic cells. It is very suitable for the transplantation of human hematopoietic stem cells (CD34+ HSCs) and peripheral blood mononuclear cells (PBMC) to obtain humanized mice with human immune system.
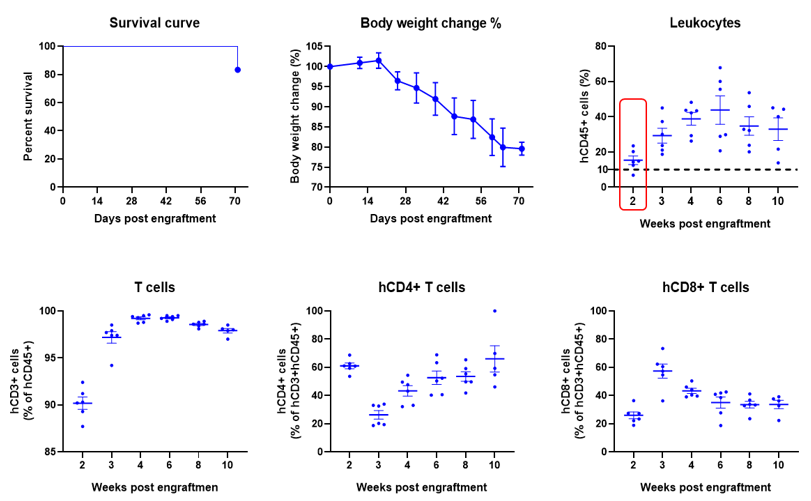
Engraftment of human PBMCs in B-NDG mice enhances reconstitution of human T cells. Human PBMCs (5E6) were intravenous engrafted into B-NDG mice (female, 6-week-old, n=6). Body weight of each mouse was recorded every week. Death data were collected every day. The peripheral blood was taken at different time points to analyze the reconstitution levels of human immune cells. B-NDG mice showed a high percentage of human CD45+ cells and T cells. B-NDG mice exhibit shortened survival and reduced body weight likely due to GvHD caused by a high percentages of human T cell reconstitution. Values are expressed as mean ± SEM.
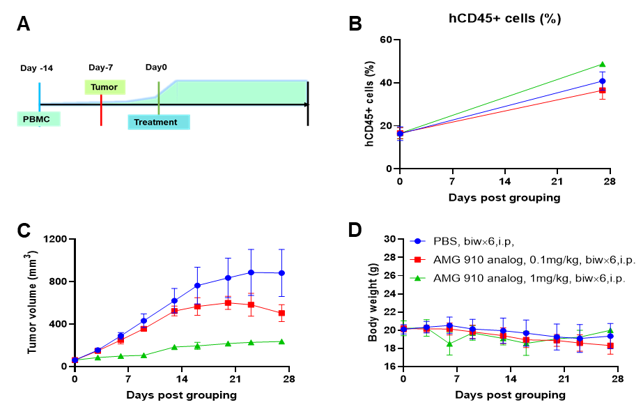
A NUGC4 human gastric cancer model was established using human PBMCs engrafted B-NDG mice and the efficacy of anti-human CD3/CLDN18.2 bispecific antibody was verified. Human PBMCs (5E6) were intravenously engrafted into B-NDG mice (female, 7-week-old, n=6). Human gastric cancer cell line NUGC4 (5E6) were inoculated subcutaneously 7 days after PBMCs engraftment. AMG 910 (in-house) was injected intraperitoneally 7 days after tumor inoculation. The animals were grouped into control and treatment when the tumor volume reached to 50-80 mm3 and the percentage of human blood hCD45+ cells ≥ 10%, at which time they were treated with drugs. (A) Schematic diagram of tumor model and drug delivery strategy; (B) Percentages of human CD45+ cells taken from peripheral blood on the day of administration (Day 0) and at the end point (Day 27); (C) Tumor volume; (D) Body weight. Results showed that the percentages of reconstituted human CD45+ cells continued to increase during administration. The anti-human CD3/CLDN18.2 bispecific antibody showed significant dose-dependent tumor suppression. B-NDG mice engrafted with human PBMCs can be used to establish tumor models and verify the efficacy of bispecific antibodies in vivo. Values are expressed as averages ± SEM.
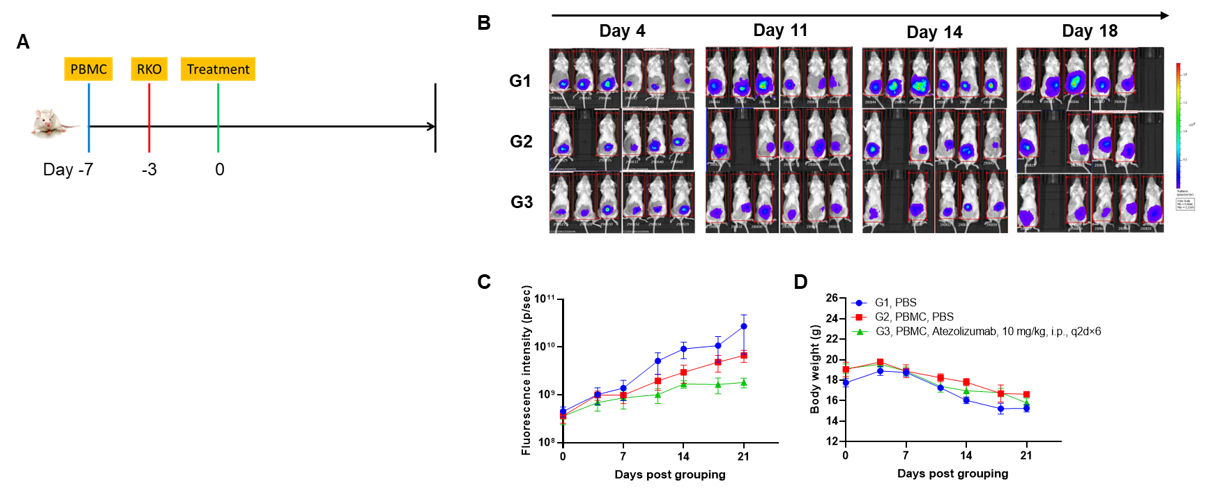
A CDX orthotopic model for human colon cancer was established using human PBMCs engrafted B-NDG mice and the efficacy of anti-human PD-L1 antibody was verified. Human PBMCs (5E6) were intravenously engrafted into B-NDG mice (female, 7-week-old, n=6). Human colonic cancer cell line B-Tg(Luc) RKO cells (1E6) were orthotopically inoculated into colonic tissue of B-NDG mice 4 days after PBMCs engraftment. Atezolizumab (in-house) was injected intraperitoneally 3 days after tumor inoculation. (A) Schematic diagram of tumor model and drug delivery strategy; (B) Imaging of mice to observe tumor growth; (C) Fluorescence intensity curve of tumor cells; (D) Body weight. Results showed that anti-human PD-L1 antibody significantly inhibited tumor growth in the orthotopic colon cancer model, demonstrating that B-NDG mice engrafted with human PBMCs can be used to establish colonic orthotopic tumor model and verify the efficacy of anti-human antibodies in vivo. Values are expressed as mean ± SEM.
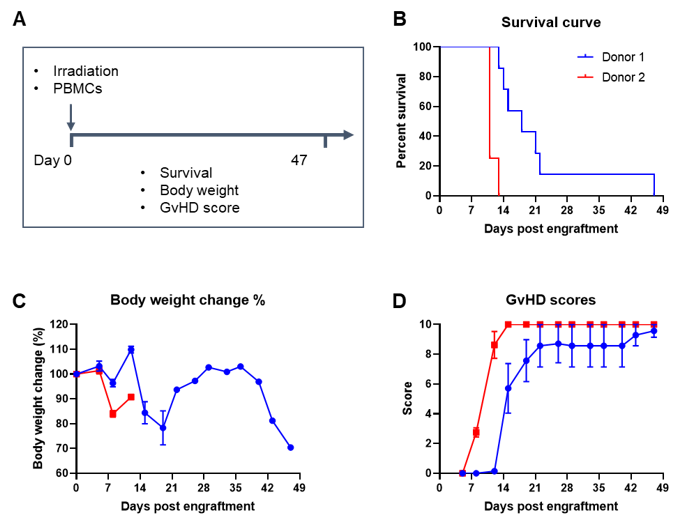
Engraftment of human PBMCs in B-NDG mice successfully constructed a graft-versus-host disease (GvHD) model. Human PBMCs (1E7) were intravenously engrafted into B-NDG mice 4 hrs after 1.0 Gy irradiation (female, 8-week-old, n=7/8). Mice were weighed twice a week and the health status of mice were recorded every day. Each mouse was scored according to the GvHD scoring standard. (A) Schematic diagram of the model-building strategy; (B) Survival curve; (C) Changes of body weight; (D) GvHD score. The results showed that the survival rate and the body weight of B-NDG mice were significantly reduced after irradiated and PBMC engraftment. GvHD scores were significant increased. But the GvHD symptoms vary between different donor sources of PBMCs. Therefore, B-NDG mice can be used to establish GvHD mouse model. Values are expressed as mean ± SEM.
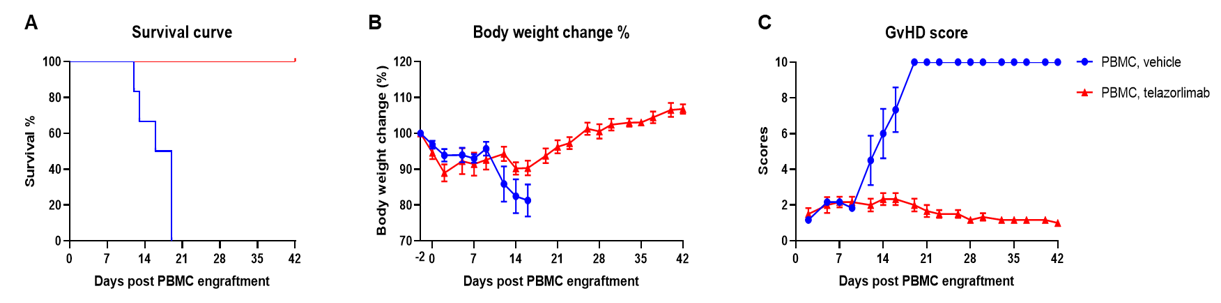
Engraftment of human PBMCs in B-NDG mice successfully constructed a graft-versus-host disease (GvHD) model and evaluated the efficacy of anti-human OX40 antibody. Human PBMCs (1E7) were intravenously engrafted into B-NDG mice 4 hrs after 1.0 Gy irradiation (female, 6-week-old, n=6). An anti-human OX40 antibody telazorlimab (in-house) was intravenously injected into the mice of the treatment group. Mice were weighed three times a week and the health status of mice were recorded every day. Each mouse was scored according to the GvHD scoring standard. (A) Survival curve; (B) Changes of body weight; (C) GvHD score. The results showed that the GvHD model was successfully constructed. The survival rate, body weight and GvHD score of mice in the treatment group (telazorlimab) were significantly improved compared with those in the vehicle group. Therefore, B-NDG mice can be used to construct GvHD model and verify the efficacy of antibodies in vivo. Values are expressed as mean ± SEM.