


C57BL/6-Cd3etm2(CD3E)Bcgen Cd28tm1(CD28)Bcgen Cd19tm4(CD19)Bcgen/Bcgen • 113092
| Product name | B-hCD3E/hCD28/hCD19 mice |
|---|---|
| Catalog number | 113092 |
| Strain name | C57BL/6-Cd3etm2(CD3E)Bcgen Cd28tm1(CD28)Bcgen Cd19tm4(CD19)Bcgen/Bcgen |
| Strain background | C57BL/6 |
| NCBI gene ID | 12487,12478 |
| Aliases | CD3E,CD3e molecule, IMD18, T3E, TCRE;CD28, CD28 molecule, Tp44; B4, CVID3 |
Gene targeting strategy for B-hCD3E/hCD28/hCD19 mice.
The exons 2-6 of mouse Cd3e gene that encode signal peptide and extracellular domain are replaced by human counterparts in B-hCD3E/hCD28/hCD19 mice. The genomic region of mouse Cd3e gene that encodes transmembrane domain and cytoplasmic portion is retained. The promoter, 5’UTR and 3’UTR region of the mouse gene are also retained. The chimeric CD3E expression is driven by endogenous mouse Cd3e promoter, while mouse Cd3e gene transcription and translation will be disrupted.
The exons 2-3 of mouse Cd28 gene that encode extracellular domain are replaced by human counterparts in B-hCD3E/hCD28/hCD19 mice. The genomic region of mouse Cd28 gene that encodes transmembrane domain and cytoplasmic portion is retained. The promoter, 5’UTR and 3’UTR region of the mouse gene are also retained. The chimeric CD28 expression is driven by endogenous mouse Cd28 promoter, while mouse Cd28 gene transcription and translation will be disrupted.
A chimeric CDS that encodes human CD19 extracellular domain, mouse CD19 transmembrane and cytoplasmic domain, followed by mouse 3’UTR-STOP is inserted right after mouse Cd19 exon 2. The chimeric CD19 protein expression will be driven by endogenous mouse Cd19 promoter, while mouse Cd19 gene transcription and translation will be disrupted.
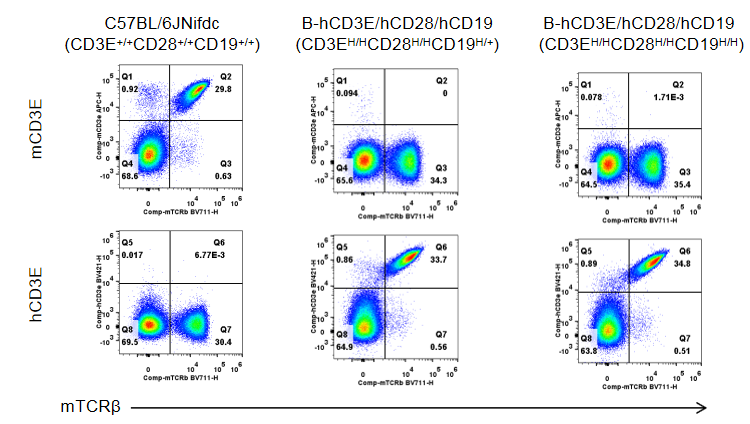
Strain specific CD3E expression analysis in wild-type C57BL/6JNifdc mice and homozygous humanized B-hCD3E/hCD28/hCD19 mice by flow cytometry.
Splenocytes were collected from wild-type C57BL/6JNifdc mice and B-hCD3E/hCD28/hCD19 mice (female, 6-week-old, n=3). Protein expression was analyzed with anti-mouse CD3E antibody (Biolegend, 100312) and anti-human CD3E antibody (BD Horizon™, 562426) by flow cytometry. Mouse CD3E was only detectable in wild-type C57BL/6JNifdc mice (CD3E+/+CD28+/+CD19+/+). Human CD3E was exclusively detectable in B-hCD3E/hCD28/hCD19 mice (CD3EH/HCD28H/HCD19H/+) and homozygous B-hCD3E/hCD28/hCD19 mice (CD3EH/HCD28H/HCD19H/H), but not in wild-type C57BL/6JNifdc mice.
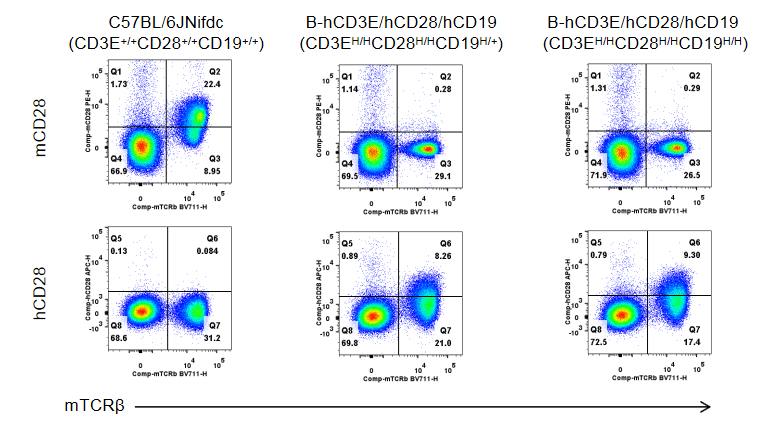
Strain specific CD28 expression analysis in wild-type C57BL/6JNifdc mice and homozygous humanized B-hCD3E/hCD28/hCD19 mice by flow cytometry.
Splenocytes were collected from wild-type C57BL/6JNifdc mice and B-hCD3E/hCD28/hCD19 mice (female, 6-week-old, n=3). Protein expression was analyzed with anti-mouse CD28 antibody (Biolegend, 102105) and anti-human CD28 antibody (Biolegend, 302912) by flow cytometry. Mouse CD28 was only detectable in wild-type C57BL/6JNifdc mice (CD3E+/+CD28+/+CD19+/+). Human CD28 was exclusively detectable in B-hCD3E/hCD28/hCD19 mice (CD3EH/HCD28H/HCD19H/+) and homozygous B-hCD3E/hCD28/hCD19 mice (CD3EH/HCD28H/HCD19H/H), but not in wild-type C57BL/6JNifdc mice.
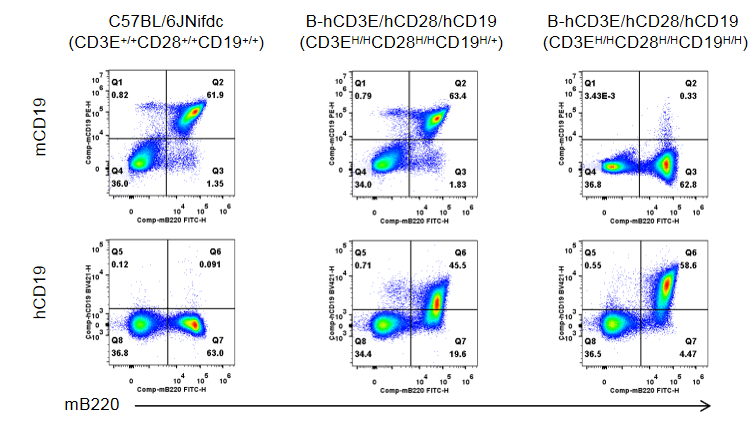
Strain specific CD19 expression analysis in wild-type C57BL/6JNifdc mice and homozygous humanized B-hCD3E/hCD28/hCD19 mice by flow cytometry.
Splenocytes were collected from wild-type C57BL/6JNifdc mice and B-hCD3E/hCD28/hCD19 mice (female, 6-week-old, n=3). Protein expression was analyzed with anti-mouse CD19 antibody (Biolegend, 115507) and anti-human CD19 antibody (Biolegend, 302234) by flow cytometry. Mouse CD19 was detectable in wild-type C57BL/6JNifdc mice (CD3E+/+CD28+/+CD19+/+) and B-hCD3E/hCD28/hCD19 mice (CD3EH/HCD28H/HCD19H/+). Human CD19 was detectable in B-hCD3E/hCD28/hCD19 mice (CD3EH/HCD28H/HCD19H/+) and homozygous B-hCD3E/hCD28/hCD19 mice (CD3EH/HCD28H/HCD19H/H), but not in wild-type C57BL/6JNifdc mice.
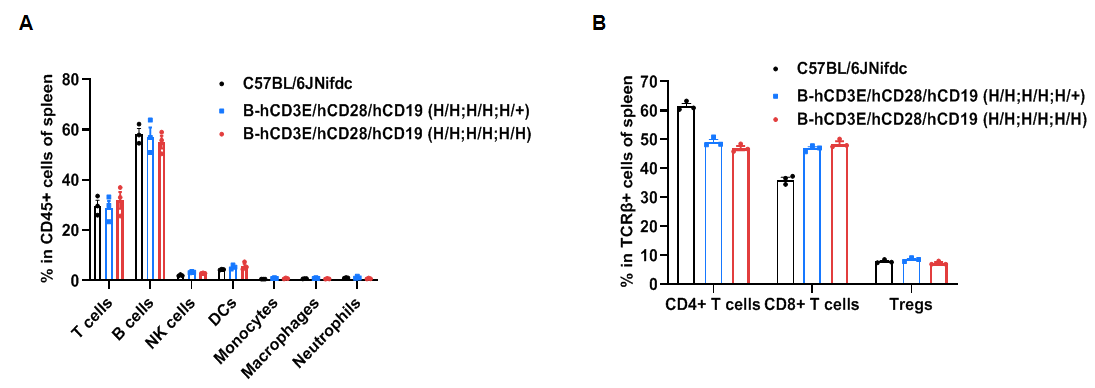
Frequency of leukocyte subpopulations in spleen by flow cytometry.
Splenocytes were isolated from wild-type C57BL/6JNifdc mice and homozygous B-hCD3E/hCD28/hCD19 mice (female, 6-week-old, n=3). A. Flow cytometry analysis of the splenocytes was performed to assess the frequency of leukocyte subpopulations. B. Frequencies of T cell subpopulations. Percentages of T cells, B cells, NK cells, DCs, monocytes, macrophages, neutrophils, CD4+ T cells, CD8+ T cells and Tregs in B-hCD3E/hCD28/hCD19 mice were similar to those in C57BL/6JNifdc mice, demonstrating that humanization of CD3E, CD28 and CD19 do not change the frequency or distribution of these cell types in spleen. Values are expressed as mean ± SEM.
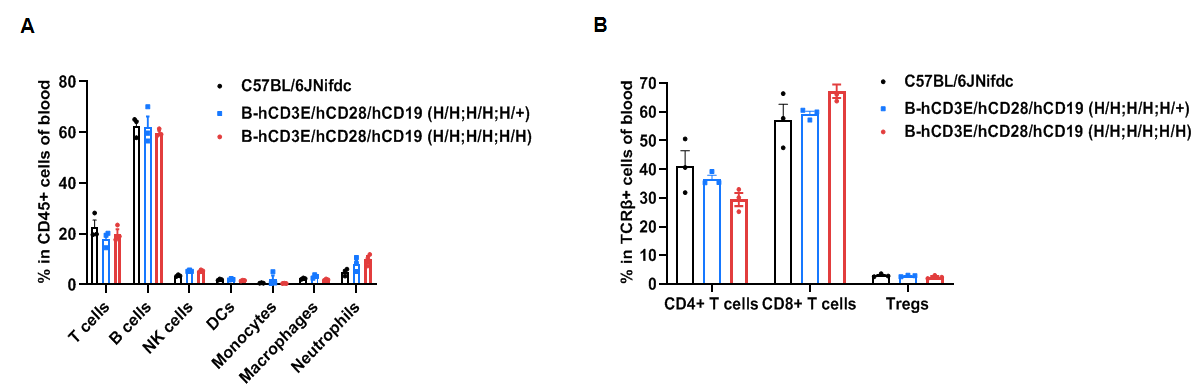
Frequency of leukocyte subpopulations in blood by flow cytometry.
Blood cells were isolated from wild-type C57BL/6JNifdc mice and homozygous B-hCD3E/hCD28/hCD19 mice (female, 6-week-old, n=3). A. Flow cytometry analysis of the blood cells was performed to assess the frequency of leukocyte subpopulations. B. Frequencies of T cell subpopulations. Percentages of T cells, B cells, NK cells, DCs, monocytes, macrophages, neutrophils, CD4+ T cells, CD8+ T cells and Tregs in B-hCD3E/hCD28/hCD19 mice were similar to those in C57BL/6JNifdc mice, demonstrating that humanization of CD3E, CD28 and CD19 do not change the frequency or distribution of these cell types in blood. Values are expressed as mean ± SEM.
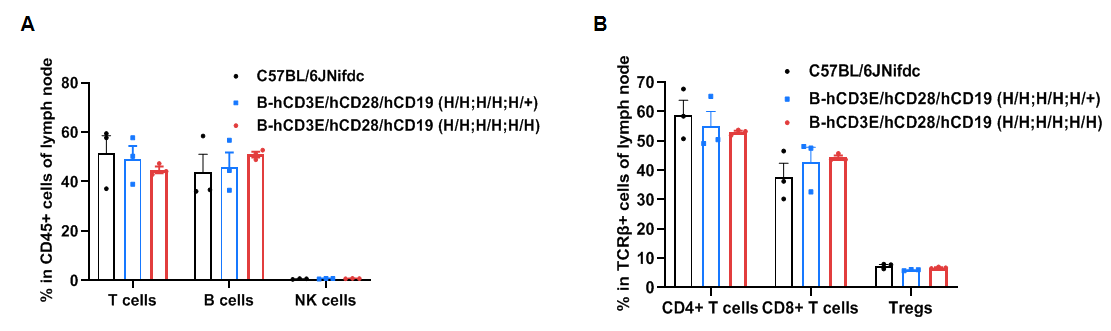
Frequency of leukocyte subpopulations in lymph node by flow cytometry.
Leukocytes were isolated from wild-type C57BL/6JNifdc mice and homozygous B-hCD3E/hCD28/hCD19 mice (female, 6-week-old, n=3). A. Flow cytometry analysis of the leukocytes was performed to assess the frequency of leukocyte subpopulations. B. Frequencies of T cell subpopulations. Percentages of T cells, B cells, NK cells, CD4+ T cells, CD8+ T cells and Tregs in B-hCD3E/hCD28/hCD19 mice were similar to those in C57BL/6JNifdc mice, demonstrating that humanization of CD3E, CD28 and CD19 do not change the frequency or distribution of these cell types in lymph node. Values are expressed as mean ± SEM.