


C57BL/6-Lrrc32tm1(LRRC32)Bcgen/Bcgen • 110102
| Product name | B-hGARP mice |
|---|---|
| Catalog number | 110102 |
| Strain name | C57BL/6-Lrrc32tm1(LRRC32)Bcgen/Bcgen |
| Strain background | C57BL/6 |
| NCBI gene ID | 434215 (Human) |
| Aliases | LRRC32 (Leucine-Rich Repeat-Containing Protein 32), GARP (Glycoprotein-A repetitions predominant) |
on this page
Gene targeting strategy for B-hGARP mice. The exon 1~2 of mouse Lrrc32 gene that encode the full-length were replaced by human LRRC32 exon 1~2 in B-hGARP mice.
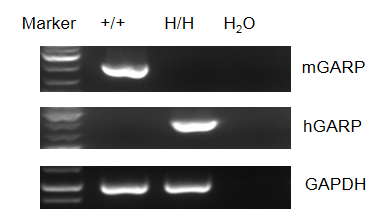
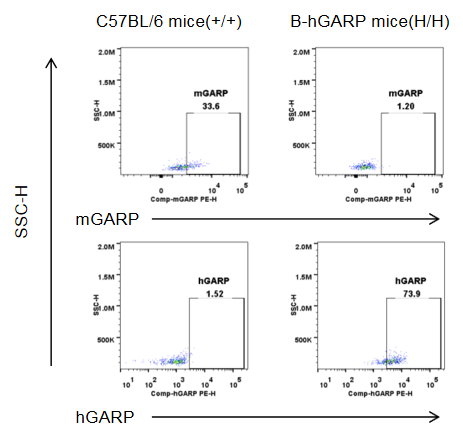
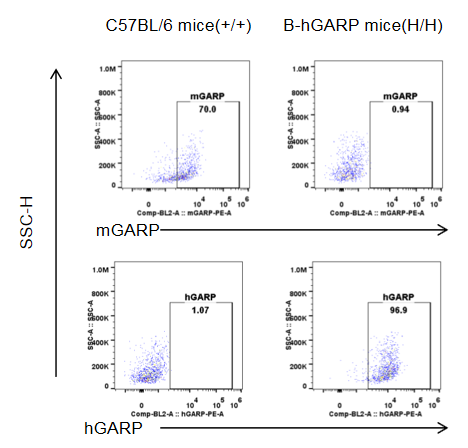
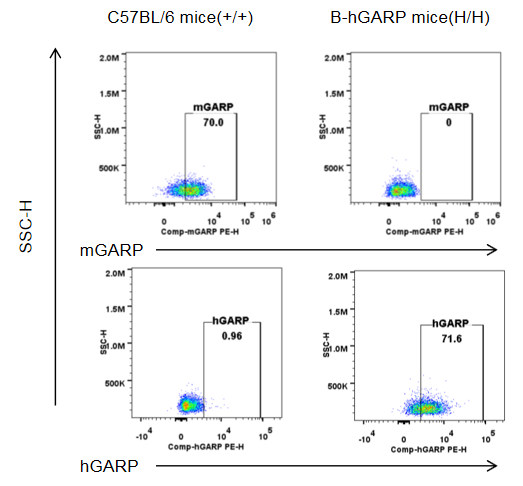
Strain specific GARP expression analysis in homozygous B-hGARP mice by flow cytometry. Blood was isolated from wild-type C57BL/6 mice and homozygous B-hGARP mice, and platelet were gated for mCD41 population and used for further analysis as indicated here. Human GARP was only detectable in homozygous homozygous B-hGARP mice.
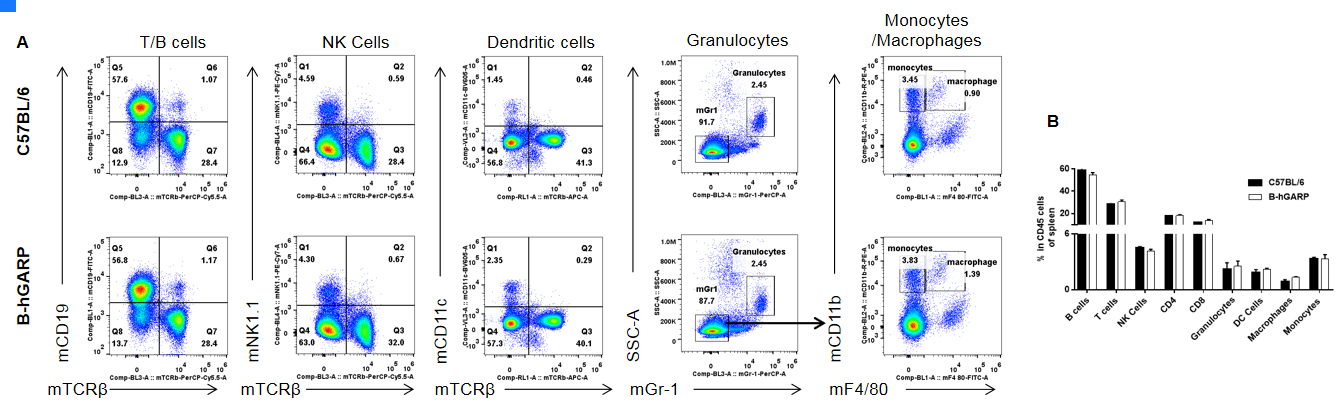
Analysis of splenic leukocyte subpopulations by FACS. Splenocytes were isolated from female C57BL/6 and B-hGARP mice (n=3, 6-week-old) and analyzed by flow cytometry to assess leukocyte subpopulations. (A) Representative FACS plots gated on single live CD45+ cells for further analysis. (B) Results of FACS analysis. Percentages of T, B, NK cells, monocytes/macrophages, and DC were similar in homozygous B-hGARP mice and C57BL/6 mice, demonstrating that introduction of hGARP in place of its mouse counterpart does not change the overall development, differentiation, or distribution of these cell types in spleen. Values are expressed as mean ± SEM.
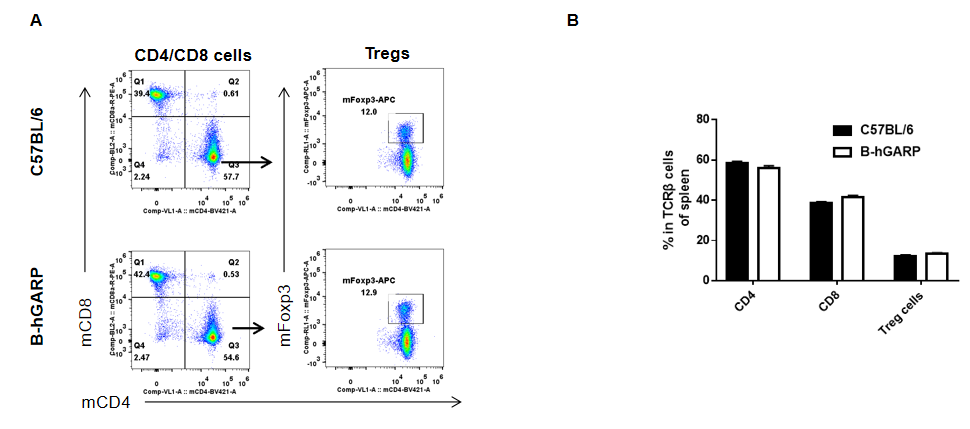
Analysis of splenic T cell subpopulations by FACS. Splenocytes were isolated from female C57BL/6 and B-hGARP mice (n=3, 6-week-old) and analyzed by flow cytometry for T cell subsets. (A) Representative FACS plots gated on CD3+ T cells and further analyzed. (B) Results of FACS analysis. Percentages of CD8+, CD4+, and Treg cells were similar in homozygous B-hGARP and C57BL/6 mice, demonstrating that introduction of hGARP in place of its mouse counterpart does not change the overall development, differentiation or distribution of these T cell subtypes in spleen. Values are expressed as mean ± SEM.
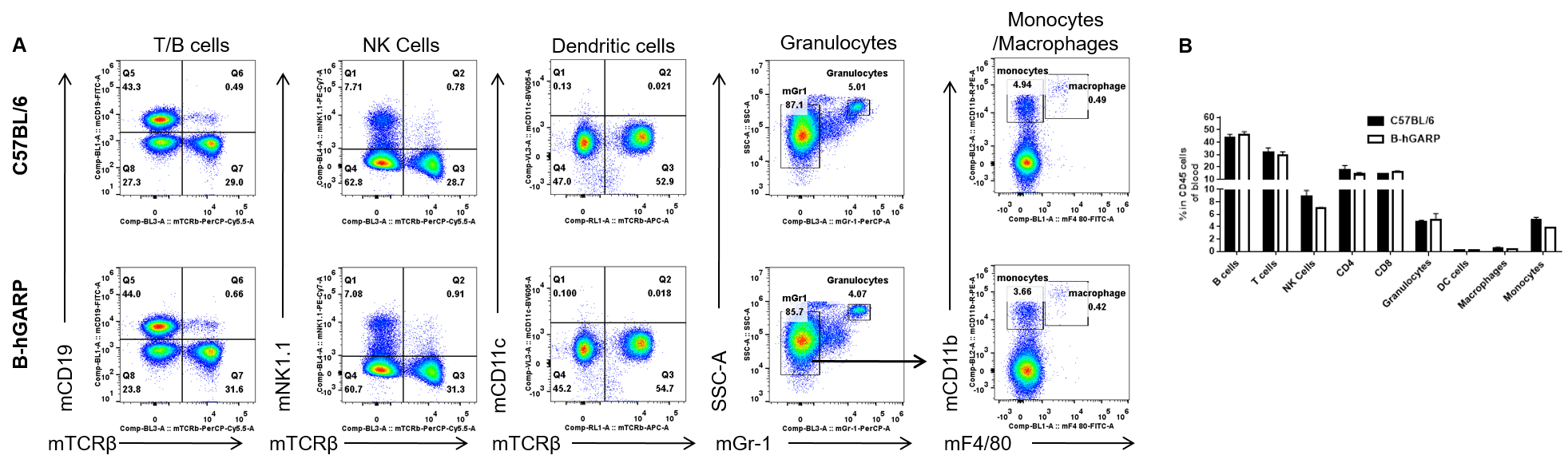
Analysis of blood leukocyte subpopulations by FACS. Blood were isolated from female C57BL/6 and B-hGARP mice (n=3, 6-week-old) and analyzed by flow cytometry to assess leukocyte subpopulations. (A) Representative FACS plots gated on single live CD45+ cells for further analysis. (B) Results of FACS analysis. Percentages of T, B, NK cells, monocyte/macrophages, and DC were similar in homozygous B-hGARP mice and C57BL/6 mice, demonstrating that introduction of hGARP in place of its mouse counterpart does not change the overall development, differentiation, or distribution of these cell types in blood. Values are expressed as mean ± SEM.
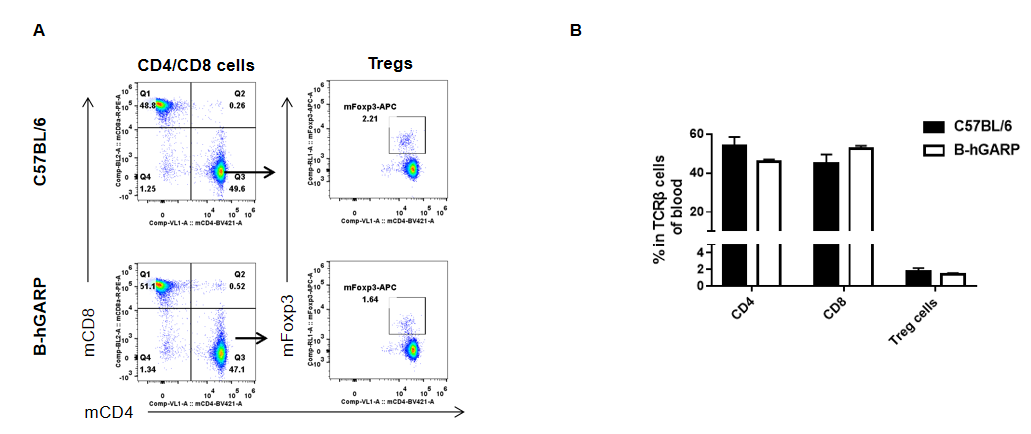
Analysis of blood T cell subpopulations by FACS. Blood were isolated from female C57BL/6 and B-hGARP mice (n=3, 6-week-old) and analyzed by flow cytometry for T cell subsets. (A) Representative FACS plots gated on CD3+ T cells and further analyzed. (B) Results of FACS analysis. Percentages of CD8+, CD4+, and Treg cells were similar in homozygous B-hGARP and C57BL/6 mice, demonstrating that introduction of hGARP in place of its mouse counterpart does not change the overall development, differentiation or distribution of these T cell subtypes in blood. Values are expressed as mean ± SEM.
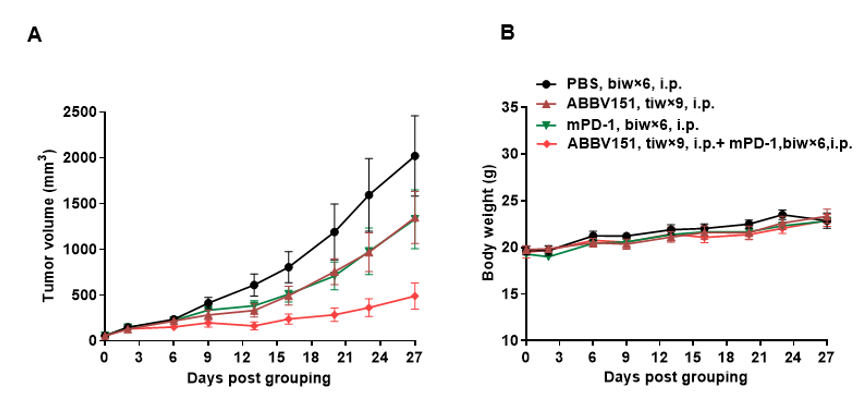
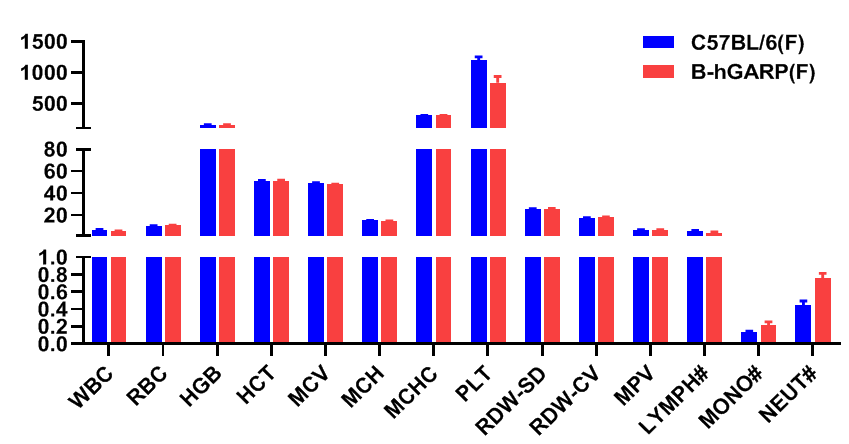
Blood routine tests of B-hGARP mice. Blood from female C57BL/6 and B-hGARP mice (n=6, 6-week-old, female) were collected and analyzed for CBC. Any measurement of B-hGARP mice in the panel were similar to C57BL/6, indicating that humanized mouse does not change blood cell composition and morphology. Values are expressed as mean ± SEM.
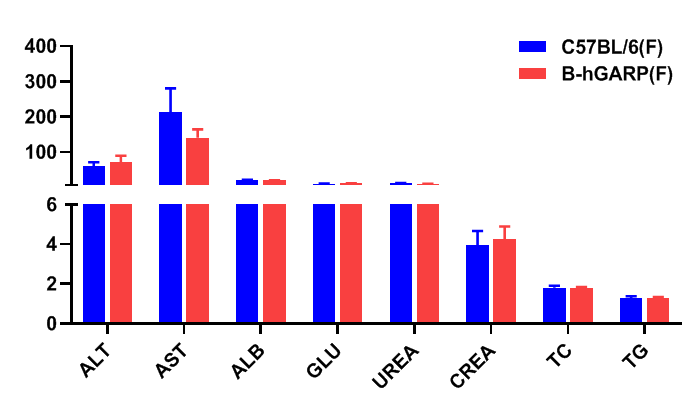
Blood chemistry tests of B-hGARP mice. Serum from C57BL/6 and B-hGARP mice (n=6, 6-week-old, female) were collected and analyzed for levels of ALT, AST and other indicators in the panel. There was no differences on either measurement between C57BL/6 and humanized mouse, indicating that humanized mouse does not change ALT and AST levels or health of liver. Values are expressed as mean ± SEM.