C57BL/6-Il21rtm1(IL21R)Bcgen/Bcgen • 110766
| Product name | B-hIL21R mice |
|---|---|
| Catalog number | 110766 |
| Strain name | C57BL/6-Il21rtm1(IL21R)Bcgen/Bcgen |
| Strain background | C57BL/6 |
| NCBI gene ID | 60504 |
| Aliases | CD360, IMD56, NILR |
on this page
IL21R encodes a cytokine receptor for interleukin 21 (IL21). It belongs to the type I cytokine receptors, and has been shown to form a heterodimeric receptor complex with the common gamma-chain, a receptor subunit also shared by the receptors for interleukin 2, 4, 7, 9, and 15. This receptor transduces the growth promoting signal of IL21, and is important for the proliferation and differentiation of T cells, B cells, and natural killer (NK) cells. The ligand binding of this receptor leads to the activation of multiple downstream signaling molecules, including JAK1, JAK3, STAT1, and STAT3. Knockout studies of a similar gene in mouse suggest a role for this gene in regulating immunoglobulin production.
Gene targeting strategy for B-hIL21R mice. The exons 3-6 of mouse Il21r gene that encode the extracellular domain were replaced by human IL21R exons 3-6 in B-hIL21R mice.
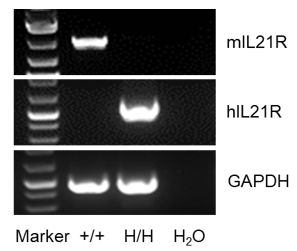
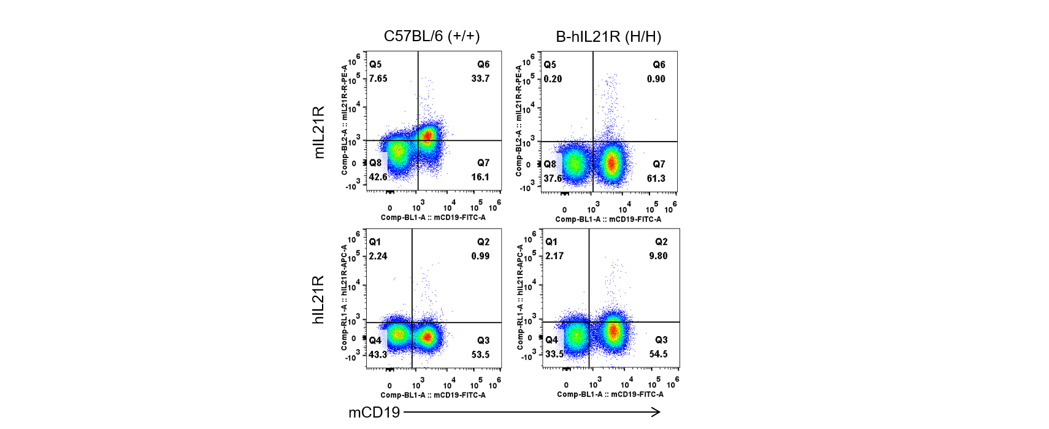
Strain specific IL21R expression analysis in homozygous B-hIL21R mice by flow cytometry. Splenocytes were collected from wild-type C57BL/6 mice (+/+) and homozygous B-hIL21R mice (H/H), and analyzed by flow cytometry with species-specific anti-IL21R antibody. Mouse IL21R was detectable in wild-type C57BL/6 mice. Human IL21R was exclusively detectable in homozygous B-hIL21R mice but not in wild-type mice.
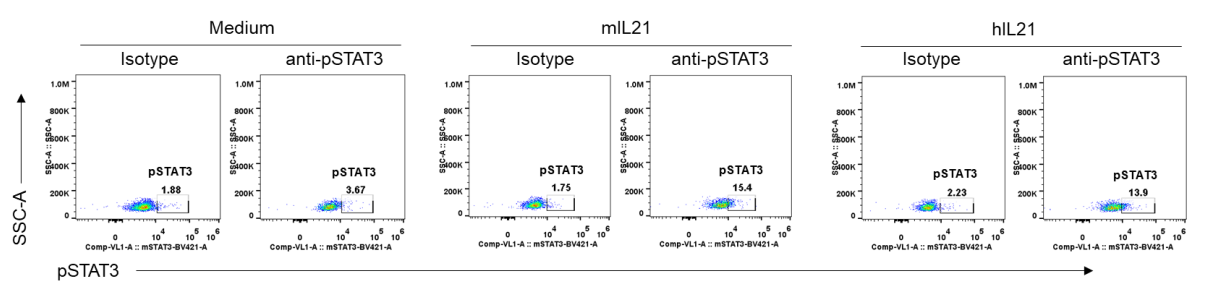
Mouse pSTAT3 was induced with mouse IL21 and human IL21 in homozygous B-hIL21R mice analyzed by flow cytometry. Splenocytes were collected from homozygous B-hIL21R mice (H/H), and stimulated with mIL21 or hIL21. The induction of STAT3 phosphorylation on CD8+ T cells with the indicated stimulators was assayed by flow cytometry. Results indicated that STAT3 phosphorylation was successfully induced with mouse IL21 or human IL21 in homozygous B-hIL21R mice.
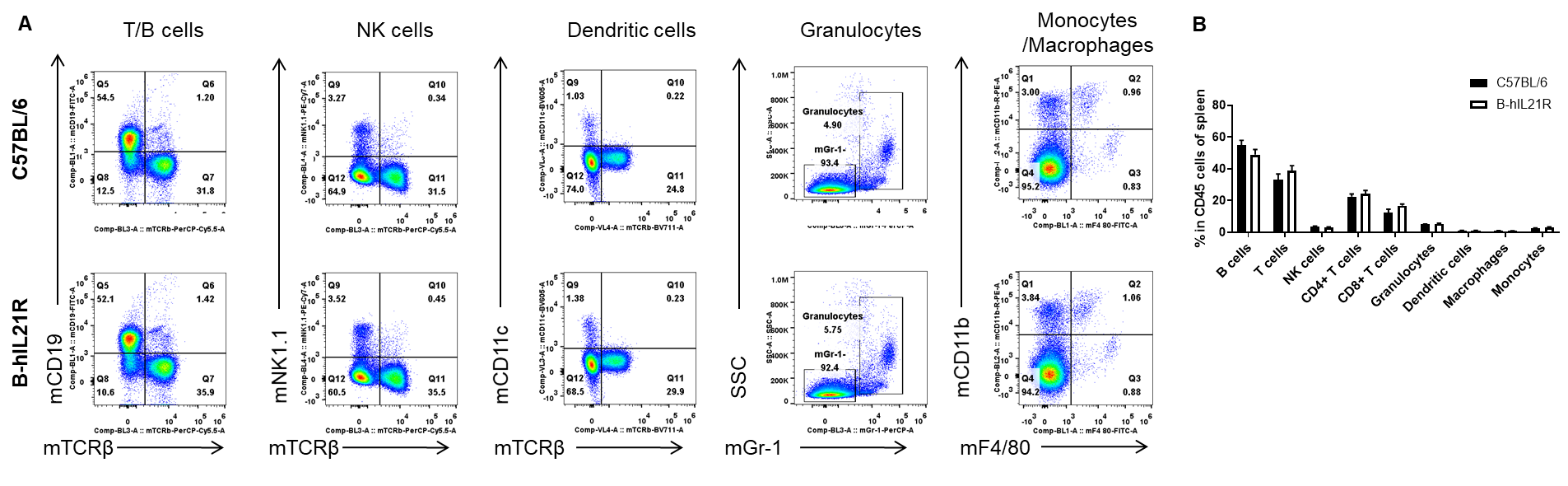
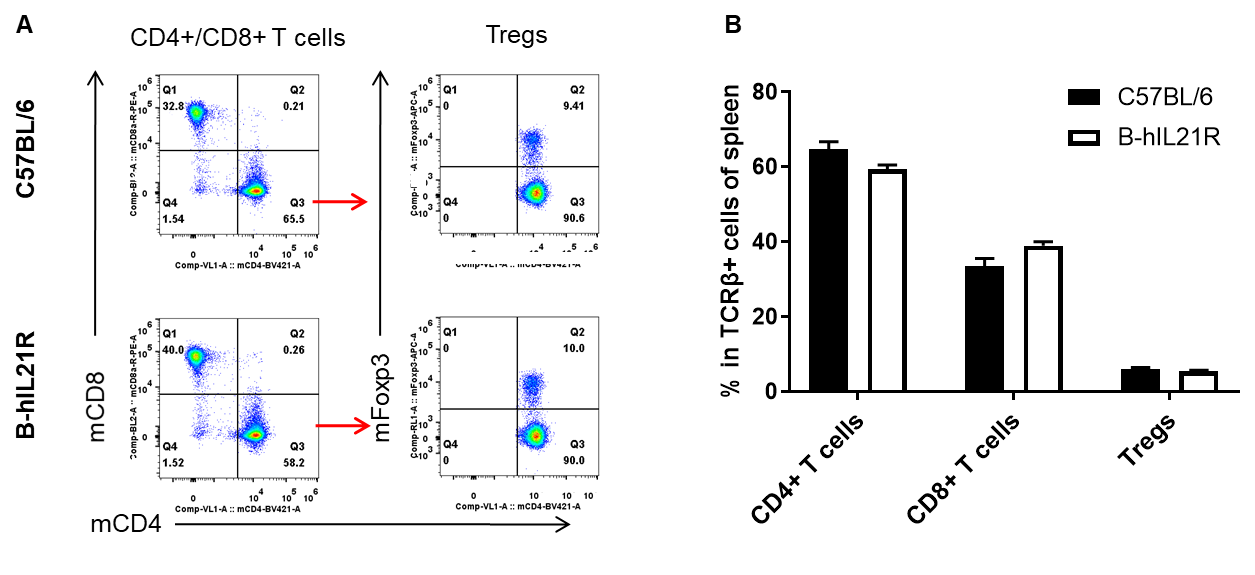
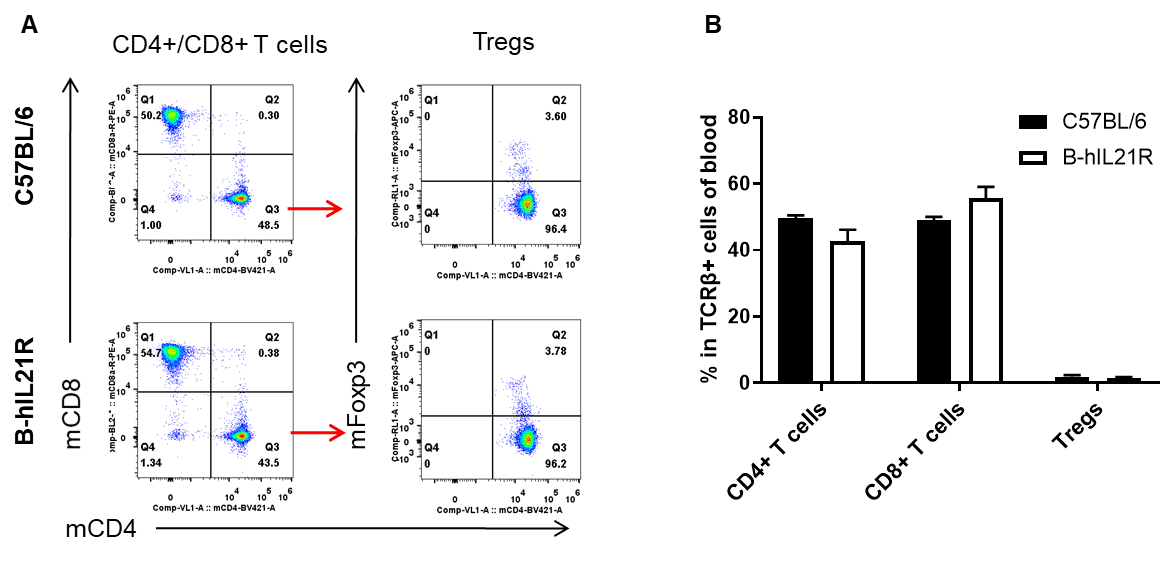
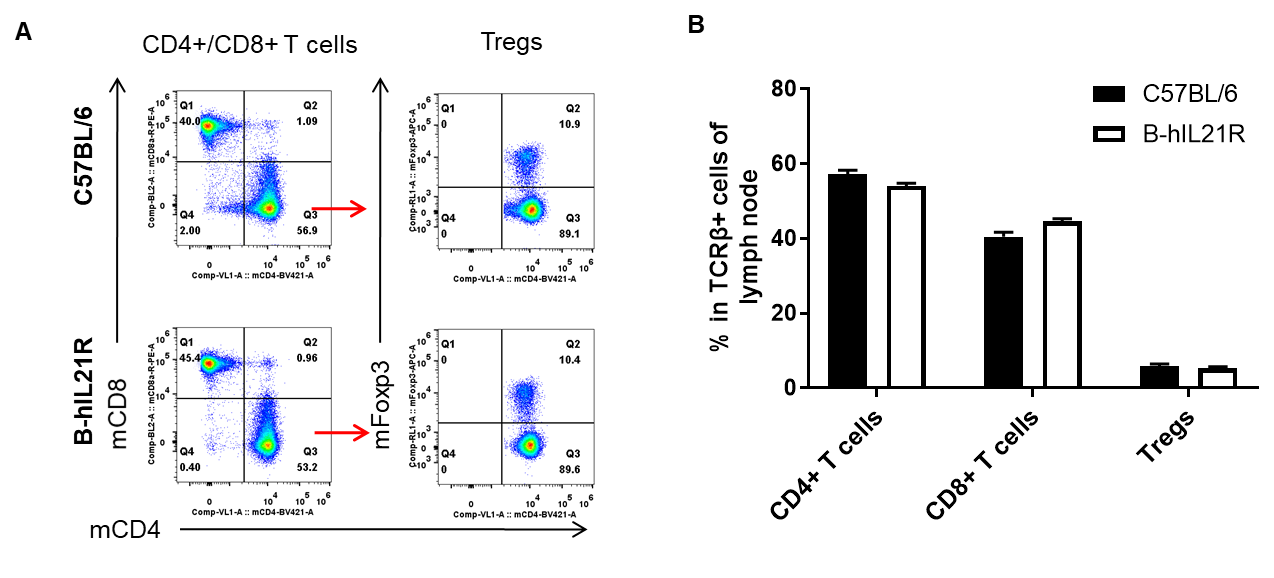
Analysis of spleen T cell subpopulations by FACS. Splenocytes were isolated from female C57BL/6 and B-hIL21R mice (n=3, 6-week-old). Flow cytometry analysis of the splenocytes was performed to assess leukocyte subpopulations. A. Representative FACS plots. Single live CD45+ cells were gated for TCRβ+ T cell population and used for further analysis as indicated here. B. Results of FACS analysis. The percent of CD4+ T cells, CD8+ T cells and Tregs in homozygous B-hIL21R mice were similar to those in the C57BL/6 mice, demonstrating that introduction of hIL21R in place of its mouse counterpart does not change the overall development, differentiation or distribution of these T cell subtypes in spleen. Values are expressed as mean ± SEM.
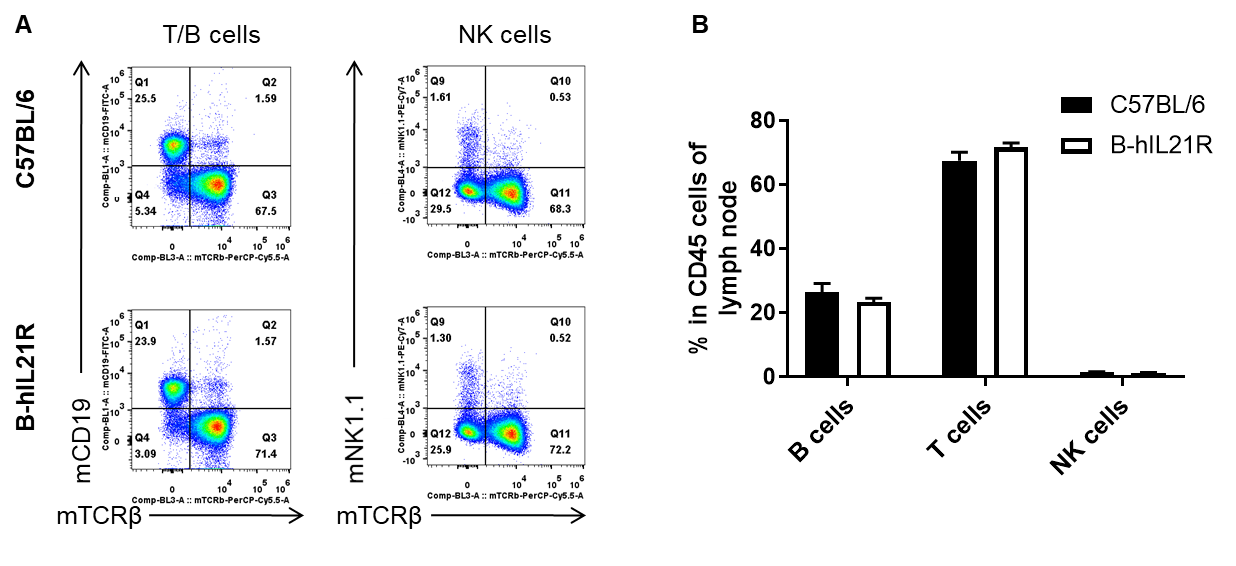
Analysis of lymph node leukocyte subpopulations by FACS. Lymph nodes were isolated from female C57BL/6 and B-hIL21R mice (n=3, 6-week-old). Flow cytometry analysis of the leukocytes was performed to assess leukocyte subpopulations. A. Representative FACS plots. Single live cells were gated for the CD45+ population and used for further analysis as indicated here. B. Results of FACS analysis. Percent of T cells, B cells and NK cells in homozygous B-hIL21R mice were similar to those in the C57BL/6 mice, demonstrating that IL21R humanized does not change the overall development, differentiation or distribution of these cell types in lymph node. Values are expressed as mean ± SEM.
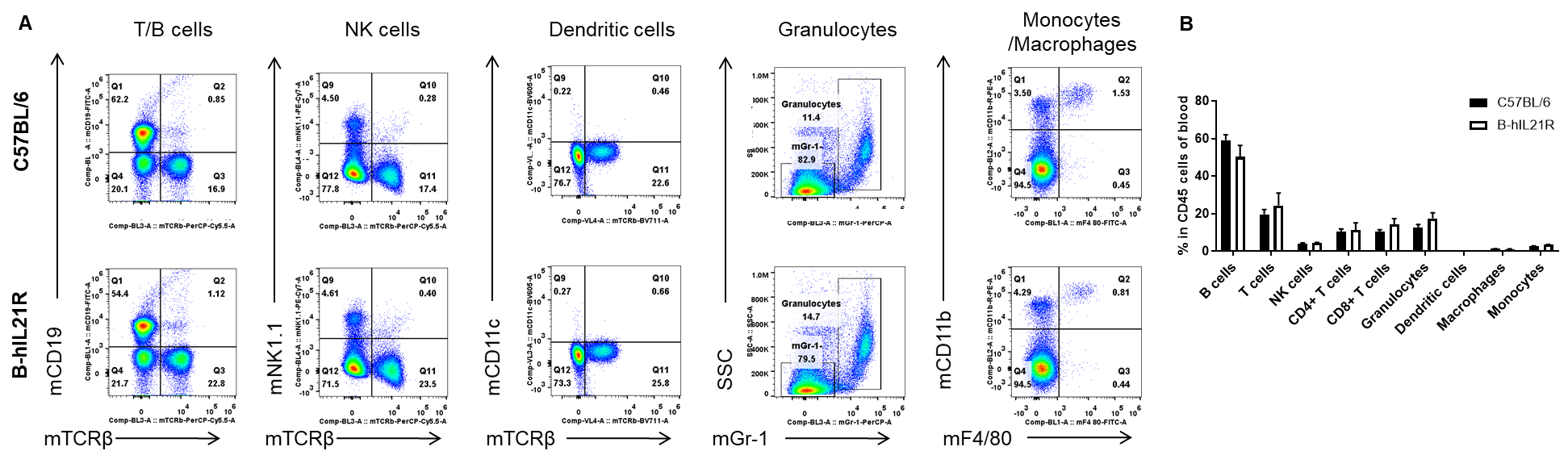
Analysis of blood leukocyte subpopulations by FACS. Blood cells were isolated from female C57BL/6 and B-hIL21R mice (n=3, 6-week-old). Flow cytometry analysis of the blood cells was performed to assess leukocyte subpopulations. A. Representative FACS plots. Single live cells were gated for the CD45+ population and used for further analysis as indicated here. B. Results of FACS analysis. Percent of T cells, B cells, NK cells, dendritic cells, granulocytes, monocytes and macrophages in homozygous B-hIL21R mice were similar to those in the C57BL/6 mice, demonstrating that IL21R humanized does not change the overall development, differentiation or distribution of these cell types in blood. Values are expressed as mean ± SEM.